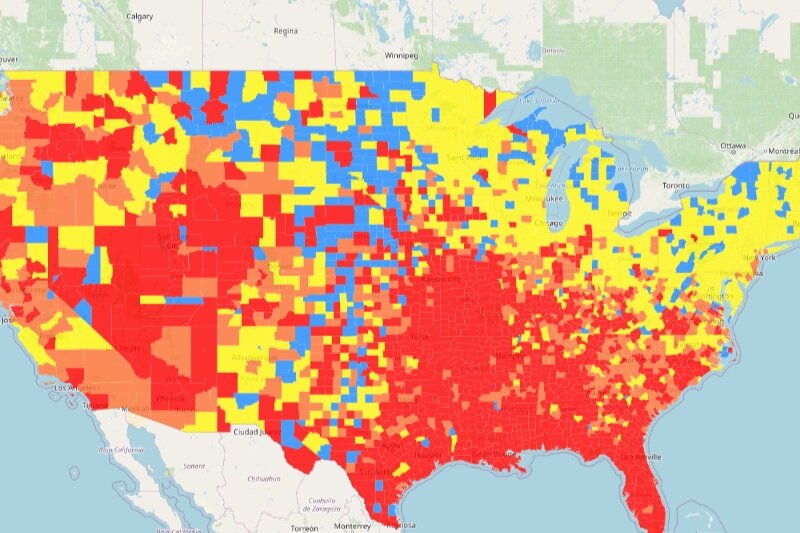
Biden Drug Policy Agenda: NIH Invests in Harm Reduction
On December 29th, 2021, the National Institute of Health (NIH) issued two new requests for application (RFA), one for the establishment of a “Harm Reduction Network” and another for a data coordination center in support of the network. The idea the NIH proposes is to develop and test new harm reduction strategies, examining the efficacy of existing harm reduction models, effective implementation of harm reduction strategies, and examining new models targeting diversified settings and delivery models of harm reduction services. The data coordination center will focus on meeting with relevant stakeholders, defining common metrics, developing research and clinical practice models, and otherwise analyzing the landscape of harm reduction across the nation. This move represents the “investigative” phase of the Biden Drug Policy Agenda.
Of note, the NIH very specifically cites interest in exploring the impacts of decriminalization and safe consumption sites as harm reduction policies and syringe service programs (including vending machines and mail programs), community based infectious disease services and prevention programs (specifically mentioning HIV and HCV), naloxone programs, and fentanyl testing strip programs.
In discussing decriminalization as a policy, much existing work is focused on marijuana decriminalization (either for medical or recreational use) in which several states have progressed in passing legislation in recent years. However, few of these pieces of legislation address people who are incarcerated currently or previous criminal records or restitution to these people for imprisonment related to possession, use, or distribution of marijuana. This has left an extraordinarily inequitable landscape with regard to marijuana as an industry – white guys are getting rich for what Black men and women are being imprisoned for. But none of this speaks to the motivation of NIH in these RFAs: reducing fatal and non-fatal overdose deaths and marijuana isn’t typically associated with these types of outcomes. Rather, state drug paraphernalia laws may be more apt at addressing these issues. For example, Louisiana’s statute outlines anything used to test a substance’s “purity” as prohibited and criminal. Decriminalization efforts should be broadly construed for applications and not just focus on particular illicit substances but also the items substance users may access to consume products safely. Indeed, being able to “test” a substance is a well-established mechanism for users to reduce potential harms.
Similarly, safe consumption sites have long faced an uphill battle in the United States due to the “crack house” provision of the Controlled Substances Act (CSA), exemplified by the legal fight Safehouse of Pennsylvania is currently facing. Safehouse argues the relevant provision of the CSA doesn’t apply to them; the language makes it a crime to own or operate a property meant for the consumption of illegal or illicit substances, Safehouse argues they operate for the purposes of saving (a religious calling protected by the Religious Freedom Restoration Act), not drug consumption. The most effective way to save lives is by offering services where they’re needed most, including overdose reversal, housing and recovery linkage to care, syringe exchange, and HIV screenings. The Office of National Control Policy has expressed support for safe consumption sites, generally speaking, but refuses to address the legal issues Safehouse is facing. The clear lack of alignment between OFNCP and the Department of Justice has left advocates more than a tad frustrated. What’s important to note about the CSA’s “crack house” provision is the reason users gather is often related both to enjoyment of experience but also safety; they’re “unsanctioned” consumption sites, as users have until recently had to rely upon their own networks for safety. Like with any issue of access to care, sanctioned safe consumption sites pose the potential to further existing health disparities. As states warm up to the idea of supervised consumption as a service to the community, policy makers and program planners need to consider those areas which exist as medical deserts may very well be the same areas in which safe consumption sites need to exist.
Biden’s drug police agenda has numerous other items of note, including strengthening protections for people with substance use histories in the labor market under the Americans with Disabilities Act, addressing the illicit and illegal drugs supply in the country, and preventing youth from engaging in drug use. Arguably, a key component missing in much of these discussions is how to protect the interests of drug users and strengthen families struggling with substance use disorder. Under the existing punitive approach, drug users are isolated from their families by way of criminal and family courts, isolating them from a core source of social support. A common refrain in recovery, “addiction is a disease of isolation”, also has decent behavioral science research support. Separating people from their families, when those families are generally well-situated to provide necessary support, operates in direct contrast to addressing the needs of a drug user and only sets them up for failure. The Biden administration needs to evaluate family strengthening policies and incentives, including education directives and best policy practices to family courts and child protection agencies as part of this effort and the NIH initiatives should consider qualifying and quantifying how policies in these areas intersect with other harm reduction efforts.
While these initiatives and this funding opportunity is a good start. The Biden administration has a long way to go to fulfilling campaign promises and we’re already twenty-five percent of the way through his first term.
Return of the Flu: Flurona, a Co-occurring Infection that is NOT
The beginning of 2022 brought about an ominous rise in COVID-19 cases as the Omicron variant began to ravage the United States in earnest, with the Centers of Disease Control and Prevention reporting about 1.3 million new cases on January 10th. While this report is inclusive of a weekend backlog, representing the majority of states’ reporting for 2 days, rather than 1, this kind of report for any respiratory transmission is truly startling. In the background, another virus with a respiratory transmission mode, influenza, had been crushed to near non-existence during the 2020-2021 season, according to the CDC’s FluView surveillance report. Indeed, on the surface, what appears to stop COVID transmission, stops flu transmission even better. But with the relaxing of mitigation measures, “pandemic fatigue”, and society eagerly looking to move on, the flu has begun to mount its seasonal return.
In comes the frightening shadow of “flurona”! Social media sites buzzed with the dire warning experts had given in 2020: a ghastly winter with two very dangerous, highly communicable diseases ripping through the nation. The difference in late 2021 and early 2022, compared to the year before, is obvious: wide access to COVID-19 vaccinations (in the United States, at least) and a continuation of annual influenza vaccination availability. This co-occurring infection, however, isn’t new. Indeed, the United States likely experienced some combined infections during the early days of the COVID pandemic in 2020, prior to the wide availability of diagnostic COVID tests, and again in the 2020-2021 flu season. While the instances may have been relatively rare due to the decrease in influenza transmission, the situation was not entirely unknown. It was, after all, the CDC’s FluView surveillance that shaped our initial tracking of community transmission of SARS-CoV-2 (the virus that causes COVID-19); the surveillance program tracks weekly reports from health care providers and local and state health departments of influenza like illness (ILI) incidence and the results of flu screenings in order to ascertain key metrics of public health response.
Let’s pause for a moment to acknowledge just how remarkable the 2020-2021 flu season was. A key measure in tracking influenza is pediatric mortality. In both the 2018-2019 and 2019-2020 flu seasons, the CDC reported 144 and 199, respectively, pediatric deaths attributed to the flu. In the 2020-2021 season, the CDC reported only 1 pediatric flu death (CDC data application). The total national percent positivity (or number of reactive tests relative to total tests administered) for influenza during the 2020-2021 flu season never crested the national baseline for the season of 2.6% positivity compared to only being about half way through the season this year and already having crested the national baseline (2.5%) for the last 5 weeks. In the 2019-2020 flu season, percent positivity for the flu crested the national baseline (2.4%) for 22 weeks.
We shouldn’t be dismissive of influenza. It is still a serious illness that hospitalizes many, especially vulnerable populations. National vaccination programs have done a great deal to help curb the potentially deadly impacts of influenza, though, schools have been known to be shut down due to flu outbreaks, including in early 2019. The idea of selective mitigation efforts coming and going in order to address outbreaks, isn’t new.
So here we are with Flurona – an incidence which may well have been happening this whole time, but because we don’t specifically track this particular co-occurring infection, we can’t say for sure. While there’s limited data on what to expect with a co-occurring flu and COVID infection, that data is a tad concerning; mortality did not necessarily increase but the symptomology of this type of situation did require frequent use of mechanical ventilation.
The catchy combined name of these viruses went…well…viral, even if only for a short period of time. As the project director for CANN’s HIV-HCV Coinfection Watch, the idea of a co-occurring viral infection didn’t surprise me. And it probably doesn’t surprise many of our readers here. The fact that it did surprise many members of the public, even after Dr. Anthony Fauci and other officials had previously mentioned the possibility, is indicative, inditing even, of how information is delivered and disseminated in today’s world. Numerous studies have been done on the amount of stress and anxiety people are experiencing in light of the COVID-19 pandemic. The CDC has also dedicated a page to “Coping with [pandemic-related] Stress” and many states have adopted mental health helplines for residents to dial into. The relationship between the public, experts, and news media is deeply damaged by practices of all parties – a busy public less interested in reading longer, more detail articles, a news media competing for clicks and attention in order to fund their outlets, and experts competing for space and importance because of outlet bias and lack of vetting have all harmed our ability to cohesively respond to the COVID-19 pandemic.
I’m not usually one to say “can’t we all just get along”, my job, in fact, is often about digging deep into spaces of disagreement or interest conflict and hammering out mutually beneficial concessions. This place we’ve found ourselves in as a society, where we’re all operating out of scarcity and competition at all costs is ultimately how we all “lose”; be it this pandemic, the next, or even in combatting long standing ills already needing address. Patient advocates and public health officials having to divert time and resources to educate patients and the public when a panic-inducing headline aimed at derailing the reader’s tasks is, in fact, derailing to multitudes of efforts to better the world around us if by sheer inability to focus on our tasks at hand.
If you’re struggling with coping with stress of the pandemic, flurona headlines, COVID variant headlines, any headlines, please, take a moment to review the National Alliance on Mental Illness (NAMI) COVID Resource and Information guide, or give them a shout on their hotline to be directed to area specific resources by calling 800-950-6264 or by clicking “chat with us” at the bottom of this page.
There’s little in this world that can’t be made a tiny bit more manageable with a snack, a nap, or a hug. Check those boxes, take a deep breath, and know you’re not alone.
Improvements to Public Health Guidelines, Despite Covid-19
2022 is off to a roaring Covid-19 start with both mainstream news and scientific outlets focusing on variant development, diversifying vaccines, and the impacts of the pandemic on various aspects of our lives. Last year, Community Access National Network opened our blog with discussing Covid-19’s Impact on HIV, HCV, and Substance Use Disorder and the theme crawled through our public policy discussions of the last year. While the topic is likely to set the frame for all variety of public health and policy throughout 2022, there is a necessity to discuss the developments in our space in spite of the distractions COVID has to offer.
Early 2021 found the Biden administration rescinding the “axe” the previous administration gave to the so-called “X” waiver, a requirement for providers to seek specified training in order to administer buprenorphine based medication assisted treatment for patients experiencing opioid use disorder. While providers and advocates hailed eliminating the X-waiver as a move toward advancing care, reports stated administration officials found problems with the rule as written, calling it “premature”. The Department of Health and Human Services (HHS) would later update treatment guidelines by way of formal notice posted to the federal register on April 28th, expanding eligibility of providers to administer the treatment when they “intend” to treat fewer than 30 patients a year. What enforcement looks like around the word “intent will be an area to watch as this area of public policy develops.
Later in the year, the Centers for Disease Control and Prevention (CDC) updated their Sexually Transmitted Infections Treatment Guidelines, the first overhaul since 2015. While the most significant updates to the guidelines are focused on the treatment of gonorrhea, an area of focus given the bacteria’s penchant for developing resistance to treatment, other highlights include aligning the guidelines with the CDCs 2020 recommendation for universal Hepatitis C screenings and adoption of the Advisory Committee on Immunization Practices (ACIP) recommendation for Human Papilloma Virus (HPV) “catch-up” vaccination schedules for people assigned male at birth. These and other additional updates were made, in part, because the CDC’s 2021 annual report found the United States facing the 6th consecutive year of STI increases.
Among ACIP’s many accomplishments in a year that found the panel meeting nearly twice as often as usual, a November meeting overshadowed by the endorsement of recommending Covid-19 vaccines for 5-11 year olds provided also found ACIP recommending universal adult Hepatitis B vaccination.
In a similar vein to the aforementioned updates (and with much rejoicing from advocates), the White House Office of National AIDS Policy “turned the lights back on” with the appointment of Harold Phillips as Director. Mr. Phillips provided an update to the National HIV/AIDS Strategy, announced in December with a focus on acknowledging structural barriers to achieving goals, including racism, stigma, and violence against transgender women. The plan, however, does not specifically outline ways to address these particularly challenging, systemic issues. President Biden also recognized World AIDS Day with a characteristically frank review of the history this country has with HIV and AIDS and the obstacles we still face in working to Ending the HIV Epidemic.
One of the last developments of 2021 included the CDC updating its clinical practice guidelines for pre-exposure prophylaxis for the prevention of HIV (PrEP). The update shifts language in such a way to encourage providers to more openly bring up the issue of PrEP with all patients rather than solely seeking to target “high-risk” populations. This move falls in-line with the efforts to reduce PrEP stigma among the broader public and, specifically, among providers. This was a particularly exciting development in light of the Food and Drug Administration’s (FDA) approval of cabotegravir (branded as Apretude) for PrEP. The long-acting injectable was first approved for the treatment of HIV in early 2021 and poses an extraordinary advancement in the potential for medication delivery mechanisms, improving adherence, and, ultimately, advancing efforts to End the Epidemic. Of note, pharmacy benefit managers, specifically CVS, anticipated this move as much as advocates and patients have. Despite a supposed commitment to investing in health equity with regard to HIV, CVS’ own “payor solutions” site boasts of the methods the entity will use seeking to delay or deny access to this and other innovative care under the need to “balance cost” with effective or curative treatments.
Looking into the new year, HHS’ annual policy report indicates the agency will seek to strengthen protections afforded to LGBTQ patients and more appropriately define discrimination in plan design, affecting patients living with HIV and HCV.
While these changes in direction and advancements in treatment are quite thrilling, advocates should be prepared to compete for space to be heard and anticipate familiar “foes” continuing to refuse to engage or finding ways to blockade access to care. Be they based in political ideal or industry priority or even from providers, patients and advocates would be better served when those who have traditionally disfavored advancing equity and access engaged in discussions on how to find the win-win for all parties. Community Access National Network remains committed to engaging stakeholders across interests in this space and looks forward to the good-faith efforts of those who seek to move these adversarial relationships to partnerships and even friendships.
2022: New Beginnings, New Changes
The Community Access National Network (CANN) ushers in a new beginning with the 2022 New Year, evidenced not only by the changing of the guard with our new President & CEO, but also with some important programmatic changes with our organization. We felt it important to share these changes with you.
Our weekly blog, previously branded as the HEAL Blog (Hepatitis Education, Advocacy & Leadership), is being repurposed to serve our broader mission “to define, promote, and improve access to healthcare services and supports for people living with HIV/AIDS and/or viral hepatitis through advocacy, education, and networking.” As such it is now the CANN Blog, and its areas of interest will focus on HIV/AIDS, viral hepatitis, substance use disorder, harm reduction, patient assistance programs (PAPs), Medicare, Medicaid, and the ongoing Covid-19 pandemic and its impact on public health. In keeping with the desire to monitor broader public health-related issues and appropriately engage stakeholders, our CANN Blog will be disseminated to a larger audience. Therefore, some of you may notice one more email in your inbox each Monday morning since we’re employing our general listserv to share the blog posts. It is our hope that you’ll deem the added email of value and thus maintain yourself on our listserv.
Additionally, our acclaimed HIV/HCV Co-Infection Watch will also be shared with our general listserv. But don’t worry, it only means one additional email each quarter! The HIV/HCV Co-Infection Watch offers a patient-centric informational portal serving three primary groups - patients, healthcare providers, and AIDS Service Organizations. The quarterly Watches are published in January, April, July, and October.
In 2022, our Groups will also be more active. Since 1996, our National ADAP Working Group (NAWG) has served as the cornerstone of CANN’s advocacy work on public policy. Whereas NAWG will continue to engage our HIV/AIDS stakeholders with monthly news updates, we will also convene periodic stakeholder meetings to discuss important issues facing the HIV community. Likewise, our Hepatitis Education, Advocacy & Leadership (HEAL) Group has served as an interactive national platform for the last decade on relevant issues facing people living with viral hepatitis. Periodic stakeholder meetings to discuss important issues facing the Hepatitis community will now complement the HEAL monthly newsletter. If you would like to join either the NAWG or HEAL listserv, then please do so using this link.
CANN will also launch its 340B Action Center this year. It is designed to provide patients with content-drive educational resources about the 340B Drug Discount Program and why the program matters to you. The importance of the 340B Program cannot be under-stated, and CANN remains committed to taking a balanced “money follows the patient” approach on the issues facing the program and advocating for needed reforms.
Finally, like most advocacy organizations, CANN is constantly evaluating whether it is safe (or not) to host in-person stakeholder meetings. Covid-19 has changed the advocacy landscape. Over the last two years our two signature meetings (Community Roundtable and Annual National Monitoring Report on HIV/HCV Co-Infection) have been hosted virtually, rather than in-person. CANN is taking a “wait and see” approach on how best to proceed in 2022 with these events. We will keep you apprised of our decision.
As we close the door on 2021 and open it for 2022, CANN looks forward to working with all of its community partners, industry partners, and you!
2021: A Year in Reflection
The end of 2021 is upon us and that makes this a timely opportunity to reflect on the work by the Community Access National Network (CANN). During an exceedingly busy news cycle, we have published fifty blogs (including this one) on a variety of topics ranging from the latest on policy and regulatory issues, as well as some personal perspectives. Our HIV-HCV Coinfection Watch and our Annual Monitoring Report tracked Hepatitis C (HCV) therapies covered under the State AIDS Drug Assistance Programs, Medicaid, Veterans Administration, as well as patient access via patient assistance programs, and other relevant news items affecting our patient community. We also conducted a community roundtable seeking to highlight the impacts of Covid-19 on public health programs aimed at addressing HIV, HCV, and substance use disorder (SUD).
Notably, CANN published the following six-part series designed to educate patients on various aspects of the 340B Drug Discount Program:
· A Patient’s Guide to 340B: Why the Program Matters to You
· A Patient’s Guide to 340B: Why Transparency Matters to You
· A Patient’s Guide to 340B: Why Accountability Matters to You
· A Patient’s Guide to 340B: Why the Decline in Charity Care Matters to You
· A Patient’s Guide to 340B: Why the Middlemen Matters to You
· A Patient’s Guide to 340B: Why Program Reform Matters to You
With Congress engaged in high-conflict communication, to abuse a euphemism, navigating public policy developments and pertinent issues to patients can be challenging. CANN remains committed to being an essential source of two-way communication, information, and education wherein patients write the narrative driving policy reforms and priorities. In this, we are ever grateful to the patients and caretakers who have engaged with us at every turn. Your stories matter and you are not alone in your experiences.
The diverse partnerships behind this work are critical to our success and as we end the year, we want to offer our gratitude to these essential partnerships, ranging from other patient advocacy organizations, public health associations, and industry partners.
The issues affecting our public health space of patient advocacy have not relented this year. Covid-19 has only emphasized the need to ensure these programs are effective and efficient while also highlighting the existing weaknesses and strengths of these programs. To be clear, the structural and pervasive drivers of health disparities have been named; racism, sexism, classism, ableism, and all other biases which reflect a moral justification for out ethical failings must be addressed in tandem with policy changes and adequate public health program funding in order for us to succeed in these fights for patient lives. Health equity cannot be meaningfully segregated from the policy mechanisms in which these disparities have survived in the face of another pandemic – when our collective awareness of these inequities and leverage to progress on these issues should have been their strongest and yet were not.
It’s with these things in mind, we want to leave you with the enduring sentiment that next year offers us yet another opportunity to approaches these challenges with fresh eyes and fresh ideas. We are indeed stronger together and we sincerely look forward to working with you all to move closer in realizing a world of greater access to care, fewer and smaller health disparities, and, ultimately, a more fair and loving environment in which to live our lives and raise our families.
Author’s note: I often end certain professional meetings with telling my colleagues “Love ya’ll”. It’s a sentiment I mean to depths of my soul. I am fortunate to work with some of the most amazing people in the world – folks who share an unbridled commitment to improving the lives of those around them. It’s from this same space I wish to offer each of you reading this a moment to breathe and the same open heartedness. I want to leave you all with a short story that has shaped me in more ways than I can count, The Perfect Heart, and an encouragement to tell someone you love them as soon as you can. May this next year be gentler with us all and find us giving away more pieces of our hearts.
FDA Move Aims to Diversify HCV Test Maker Market, Reduce Regulatory Burden
On November 19th, the Food and Drug Administration (FDA) released a pair of orders affecting Hepatitis C (HCV) antibody screening and diagnostic tools. The move was announced as a proposed order in April 2020 with a total of 13 comments on the proposed orders, including the National Association of State and Territorial AIDS Directors (NASTAD), Hawaii and Washington state Departments of Health, the National Hepatitis Roundtable, and one of the current leading device manufacturers, Abbott.
The final orders reclassify already existing screening and confirmatory blood and plasma testing while also changing the name of acknowledged name of the technology in use from “assay devices” to “tests”. The move is anticipated to reduce the burden in applying for regulatory approval of new tests but also require “special controls” to ensure the quality of testing technologies on the market continue to meet the high safety and accuracy standards they currently meet.
Of note, the FDA considered the impact the new final orders would have on public health initiatives, specifically the National Viral Hepatitis Strategic Plan. Additionally, Abbott’s support comment was relatively brief and contained only the concern for possible product code labeling limitations for potential technologies; suggesting the FDA take a similar approach as taken to combination antibody and antigen testing with regard to HCV as with HIV testing. Abbott’s recommendation in the comment was to merely name the technologies “serological” tests rather than limiting application based on the mechanism of action in the test as both the proposed and final orders did.
NASTAD’s comment highlighted the difficulty in current surveillance efforts as confirming an acute or active HCV infection is a two-step process, of which, the organization claims few providers or patients follow through. NASTAD drew direct and natural and logical conclusions from the Centers for Disease Control and Prevention’s (CDC’s) Viral Hepatitis Surveillance Report, highlighting the public health risk HCV poses and the barriers to more effective surveillance efforts, including the cost and regulatory hurdles for new screening technologies to enter the market. The public health interests commenting requested the FDA consider expanding the final rule to include potential future technologies like over the counter (OTC) antibody tests or screening technologies based on body fluids other than blood and plasma or combination testing technologies targeting multiple types of viral hepatitis or HCV-HIV combination tests. The FDA’s response to comments in the final orders stated the new final order could not go beyond the scope of existing technologies and, as such, would not be able to change the approval pathway for new or emerging technologies.
While the orders represent an encouragement for manufacturers to enter the existing technologies market with regard to HCV screenings, diversifying the field, the announcement does not affect developing or new technologies and the pathways to approval those potential products will need to navigate.
Jen’s Half Cents: Stepping into the Shadow of a Giant
I’ve often tried to distinguish between the sense of grief and shock that follows the death of a hero. Emotions are complex…layered especially when it comes to grief and the amount of work that’s involved in mourning and appropriate appreciation for a person’s life. When a young person dies unexpectedly, the sense of shock layered onto grief is exorbitant. That shock is expected to be less so when a person of advanced age moves on from this plane. This sentiment is not to disregard the tragedy of loss but to distinguish between “heavy” and “sharp” pains.
When William “Bill” Arnold passed on September 29th, I didn’t expect to feel the deep sense of shock that struck my heart. Tragedy, yes, because losing the presence of this titan is indeed a tragic loss for the community of advocates his work and passion shaped. But the shock was unexpected. There’s this thing that happens when admiration is a core part of one’s perception of a person – regardless of age, the relationship seems to defy necessary acknowledgments of nature even if, as was the case with Bill, the subject of said admiration challenged the admirer to not forget. In writing my second “Half Cents” this year, Bill appreciated the emphasis on needing to shore up the advocacy pipeline. After all, the blog was based in part on the very first discussion he and I shared. He cautioned, however, “there’s an obvious target here”, referring to himself. A stark reminder of his age. I think…I should have taken more note of the comment and in retrospect I regret not seeking more of an opportunity to discuss how he felt about the piece and what it meant for him, personally.
Writing this blog is a particular challenge in that few words can express the deeply complex set of feelings I’ve had to navigate since Bill’s death. The last in-person conversation Bill and I had was outside of a restaurant, after a fireside chat hosted by CANN’s sister organization, ADAP Advocacy Association in early December of 2019. As Bill and I were among the few smokers in the crowd, I enjoyed spending time with him outside, even in the bitter cold New Jersey had to offer that evening. We didn’t talk about recent politics or the upcoming election or even particular policy issues the event focused on earlier in the day, as was more common of our “smoker’s chats”. Instead, we talked about his childhood and the quite remarkable, yet humble life Bill lead. I like to remind advocates we should “bleed a little” in our work because the lives we seek to impact and the stories that drive this work are the emotional blood bonds of effective advocacy. It is unjust to expect patients to share the intimate aspects of their lives and health and to not return the favor. And in those moments, what Bill chose to share with me felt less like bleeding a bit of himself and more like welcoming me into his home. I don’t know how to reconcile that quite yet.
The greatest tragedy of death, however, is the world does not stop for grief. Things still need to be done in order for the world to function, work does not stop piling up, decisions must be made in order to not compound the difficulty of an already difficult time. In that respect, CANN’s general consultant, Brandon M. Macsata was tasked with making a recommendation of succession of Bill’s duties to CANN as President and Chief Executive Officer to the organization. This was not an easy thing. Bill had been a mentor and friend to Brandon for more than 20 years and the enormity of the moment weighed on him. He wanted to fulfill his responsibility to the organization and do justice to the Bill’s legacy, as did CANN’s Board of Directors. In this space, while we may not all always be friends, we are all comrades in a fight for improving access to care and thus the lives of those around us, in particular, the most vulnerable of our shared communities. This decision was both professional and personal, as it should be, in such intimate work.
So, when the Board of Directors, through Brandon, approached me to ask if I would be interested in assuming the role of President and Chief Executive Officer, stepping into Bill’s shadow, I had to take a moment. “These are mighty big shoes to fill”, I’d say before expressing some trepidation, not at my skill or ability, but because of an earnest desire to ensure Bill’s legacy would be appropriately honored - the Board, our patient community, and our partners would be proud of the work that follows. In expressing confidence and navigating the decision-making process, CANN’s board members placed an emphasis on both skill and temperament, a need to focus on policy changes from the patient perspective, and for the next chapter of leadership for the organization to balance these qualities and ideals.
“You and Bill are cast from the same mold”, will forever be one of the greatest compliments I will ever receive. It’s one I hold dear to my heart and the sentiment provides me a laser focus the mission at hand.
The outpouring of support CANN has received, both in offering condolences and in appointing me to the role of President and Chief Executive officer, has been incredible. While changes are certain for CANN’s future, the organization will continue with the same goals it was founded upon and has served for the 25 years; “defining, promoting, and improving access to healthcare services and supports for people living with HIV/AIDS and/or viral hepatitis through advocacy, education, and networking.”
A Patient’s Guide to 340B: Why Program Reform Matters to You
***This is the final report in a six-part series to educate patients about the 340B Drug Pricing Program***
The 340B Drug Pricing Program has no doubt added benefit for patients and providers, alike. The measure of this benefit, however, is shrouded by uncertainty over the lack of transparency and accountability, decline in hospital charity care, as well as the explosive middleman growth in contract pharmacies and pharmacy benefit managers. Twenty-nine years after the program’s inception, it is now unclear to both regulators and patients, both qualitatively and quantitatively, if the Congressional intent is being met.
With all the noise around whether rebate programs might encourage pharmaceutical manufacturers to raise the cost of their products, there is no conversation on how those rebate dollars are used. The lack of the requisite transparency reporting among non-federal grantee covered entities participating in the 340B program makes it impossible to distinguish between anecdotal claims of abuses versus legitimate use of these rebate dollars to the benefit of patients.
These combined situations place the future of the 340B program at exceptional risk, if only by politicization of the national conversation on medication affordability alone. That national conversation churns now, as Congress debates drug pricing legislation. Aside from notorious stump speeches about the prices other countries pay for their medications, nowhere in these discussions do we talk about payers (insurers) and the middleman dictating the at-the-counter prices of medications realized by patients. The ongoing political debate is absent of the larger impacts on safety-net programs benefitting from 340B revenue and the impact on the poorest patients among us. Without clear guidance, all patients can come to expect is more squabbling among covered entities, drug manufacturers, hospitals, and regulators. It is this type of environment in which an idealistic program finds itself at risk.
Lawmakers have reasonably argued federal regulators have not demonstrated a particular need for additional regulatory powers because the Health Services and Resources Administration (HRSA) has not adequately flexed their current oversight muscle (…much less that such would be exercised efficiently). Therefore, regulatory interpretation should be updated, specifically regarding the patient definition, and possibly with further defining “low-income” for more clarity on who the program should benefit most. To the extent of “cracking open the legislation”, there is a singular area in which lawmakers from both sides and the Biden Administration agree: the issue of transparency in reporting. Earlier this year, the Biden Administration’s discretionary budget included an ask of Congress to specifically fund greater oversight and administration of 340B, explicitly including requirements on reporting of how non-grantee entities use these dollars. In this space, where few agreements can be made found, this is one area where legislators can and should move swiftly. The data generated by transparent reporting on use of these dollars is invaluable in evaluating the efficacy of 340B in benefitting patients or otherwise meeting the intent of the program.
To the extent HRSA may need more room for rulemaking, legislators desperately need extend rulemaking authority to include allowable uses for 340B dollars and clarity on the intent of the program. Federal grantees already have to report use of these dollars while other covered entities aren’t. With executives reaping in millions of dollars, reasonable people can grow concerned these dollars are being used to prop up the profiteering and personal enrichment administrators may be enjoying at the expense of employees providing care and patients themselves. Employees of federal grantees don’t generally get to enjoy much in the way of raises and their pay is not on par with the private sector. Hardware and software systems lag in terms of keeping up with modern technology. Sustaining non-revenue generating or underfunded patient benefit programs is absolutely something many entities enjoy as a use of their 340B dollars. There is no doubt these dollars can be used to patient benefit beyond directly sharing the savings with patients, though sharing the savings is the most direct means patients benefit from 340B. Putting guardrails on allowable uses of these dollars would serve well everyone touched by the program. Frankly, anyone fighting this transparency as a suggested method of shoring up 340B in meeting its intended purpose has something to hide and deserves closer scrutiny.
As an additional area of critical need to consider, for non-grantee covered entity hospitals, records of charity care and minimum realized values in served communities should be determinative for qualification to participate as a covered entity. The current calculation of disproportionate share hospitals as 340B participants or non-340B participants by the Government Accountability Office has shown a steadier and steeper decline of charity care among 340B hospitals than among non-340B hospitals. Additionally, hospitals carry the highest issuance of medical debt in the United States, disproportionately affecting low-income patients. Part of ensuring low-income patients get the most benefit from the discount drug program was and remains the ability to extend no- and low-cost care, writing off costs of providing that care, without punishing patients for having a need. If hospitals are to receive the benefit of this program, that same benefit should be extended to patients.
Lastly, in addressing the sheer size of the 340B discount drug program, the most significant areas of growth with questionable benefit to patients are among contract pharmacies. HRSA’s recognized this potential in commentary with its 2010 final rule only to realize those cautionary concerns and integrate guidance curbing the use and growth of contract pharmacies in the no-shelved 2015 “mega-guidance”. While the mega-guidance has been shelved, the abuse of the program by contract pharmacies has not abated. Among reducing the number of contract pharmacies a covered entity may make agreements with, and other geographic requirements, lawmakers and regulators should consider establishing market appropriate flat fees associated with services and a database of fees charged by pharmacy benefits managers, contract pharmacies, and third-party administrators, similar to the 340B ceiling price database established under the Office of Pharmacy Affairs Information System. A similarly situated claims hub would also allow for greater clarity in audits, assessment of potential duplicate discounts, and (if appropriately structured and compliant with patient privacy laws) detect potential diversion.
340B is a massive program which, arguably, has not yet been realized by much of the patient population. Not doing anything in this case doesn’t mean “keeping things status quo”, rather it means leaving the program open to attack, inefficiency, ineffectiveness, and abuse. We can and should do more to ensure patients are aware of the program, how the program is used by covered entities nearest to them, and how this critical support to federally funded health care programs might be impacted by additional health care policy reform efforts. If ensuring the health and well-being of the country is the priority of all players in this system, then its time patients know it.
For more information on the issues facing the 340B Program, you can access the Community Access National Network’s 340B Commission final report and reform recommendations here.
Sources:
Community Access National Network (February 2019). 340B DRUG DISCOUNT PROGRAM: The Issues Spurring Discussion, Stakeholder Stances and Possible Resolutions. 340B Commission Final Report. Retrieved online at https://docs.google.com/gview?url=http://www.tiicann.org/pdf-docs/2019_CANN_340B_Commission_Final-Report-v5_03-07-19.pdf&embedded=true
A Patient’s Guide to 340B: Why the Middlemen Matters to You
***This is the fifth report in a six-part series to educate patients about the 340B Drug Pricing Program***
When the 340B Drug Pricing Program was enacted in 1992, there were a few “gaps” between the law’s statutory language and the program’s practical application. Among them was the realization that some covered entities that couldn’t afford to operate their own pharmacy. The Health Resources and Services Administration (HRSA) issued guidance to address the gap. After all, what’s the use of a discount drug program if providers can’t realize those discounts simply because they don’t have a pharmacy?
In 1996, after the urging of some covered entities, HRSA issued guidance telling covered entities and manufacturers that covered entities could contract with a single, independent pharmacy to provide pharmacy services necessary to engage the discount program. The idea was simple: create an access pipeline to the program, so it could be accessed by small providers, but not abused. In 2001, HRSA began to allow a few pilot projects, for lack of a better term, wherein covered entities would have more than one contract pharmacy. In theory, it isn’t a bad idea. Different pharmacies have different distributors, and as such supply can sometimes be an issue (i.e., natural disasters).
Additionally, it allows industrious covered entities to open the door for competition on “value added” services from contract pharmacies – such as programmatic record keeping for the purposes of 340B and/or financial reporting for federal grantees. And since the pharmacy was the one handling the purchasing and distribution of the medications to patients, that’s one less labor task for smaller covered entities to fund. In 2010, HRSA would later expand these pilot project allowance for multiple contract pharmacies per covered entity.
Sounds great, right? More patients have access to discounted outpatient medications, right?
Right? Not exactly!
Under the 340B program, patients don’t always get their share of the savings from the rebates and discounts. Arguably, it would appear everyone is directly benefiting one way or another from the program and its lucrative revenue stream, except for patients.
Contract pharmacies all want their piece of this pie, too. For example, take the dispensing fees that a pharmacy charges to fill a prescription medication. Indeed, dispensing fees for 340B contact pharmacies are so wildly non-standard a Government Accountability Office (GAO) report from 2018 found dispensing fees ranging from $0 to almost $2000 per fill on 340B eligible drugs. Those fees come out of 340B revenue, which could be supporting a patient’s ability to pay copays or the cost of a drug and instead.
Can you imagine, if you will, you’re a person living with HIV or Hepatitis C, living at about 200% of the Federal Poverty Level (FPL; 200% in 2021 is approximately $25,760 per year for a single person), but thankfully receiving insurance coverage for your medical care. Yet, co-pays and deductibles drain your finances when you could be getting your medications at no cost if the pharmacy or covered entity was applying 340B dollars to your bill? How many Rx fills would that be?
If the payer wasn’t applying a co-pay accumulator or co-pay maximizer program, the dispensing fee of two fills could mean extending your ability to access care for an entire coverage year – not just for medications, but for all health care. If the intent behind the 340B program is to extend limited federal resources, ensuring those exorbitant dispensing fees weren’t so exorbitant would certainly be one way to do it. Ultimately, 340B is a pie – when there’s more taken out, hacked at along the payment pipeline of getting medications to patients, there’s fewer resources left for patients to benefit from.
What’s more concerning about the explosive growth in the number of contract pharmacies with their hands in the 340B cookie jar, is HRSA knew when the 2010 guidance was issued that diversion and duplicate discount increases, abuses of the program, would most certainly follow. In part, because the program would grow and at such a pace that HRSA couldn’t keep up. In fact, GAO included that warning in a 2011 report, stating “…increased use of the 340B Program by contract pharmacies and hospitals may result in a greater risk of drug diversion, further heightening concerns about HRSA’s reliance on participants’ self-policing to oversee the program.”
The best part? By the “best”, I mean the worst: contract pharmacies, like non-grantee hospital entities, don’t have to show any benefit to patients for any of the dollars. Clearly, it raises questions over the legislative intent of the program and whether it is being met?
Now, contract pharmacies, like hospitals, like to massage and carefully select data to pitch answers to these concerns (there are a great number of “concerns”) by saying “we served X many 340B eligible patients”. They get around having to say if those patients realized any of those savings and benefitted from the program, without defining what they mean by “eligible”, and without defining “patient”. Contract pharmacies and hospitals get away with not having to provide meaningful information because statutory language doesn’t define “low-income” or “eligible” and regulatory guidance has an outdated definition of “patient”. Regardless of the existing language in regulation, a bona fide relationship should exist in order to call a consumer a “patient”, otherwise this is all just pocketing dollars meant for extending medication access to needy people.
All this lack of transparency fees assessed against the program could easily be solved with merely requiring contract pharmacies to establish a “flat”, reasonable dispensing fee and to describe what those fees actually cover. If the contract pharmacy is providing an additional navigation benefit to patients or an in-house location for a federally qualified health center, reasonable people can see fees being slightly elevated to cover additional costs. However, those costs should be outlined like any other contractor would be expected to do in any other contract for service. Most hospitals already have their own in-house pharmacy, they shouldn’t be contracting that service out and thus giving room for inappropriate 340B related rebate claims. And if HRSA just does not have the capacity to meaningfully audit 340B claims and the use of these dollars, they could at the very least make more room for the other mechanism in the statute for audit: manufacturer-originated audits. That’s right. The statutory language of 340B anticipated HRSA wouldn’t be able to keep up if the program was successful or even particularly abused. So, legislators reasoned if manufacturers were taking a cut of their potential profits through discounts and rebates, manufacturers should be able to audit the claims seeking those discounts and rebates to make sure everything was in line. When a retailer offers a discount to veterans, they typically require proof of veteran status. Why would medication discounts be any different?
In the end, if contract pharmacies don’t have anything to hide, then they need to stop hiding so very much. There are enough hands in the 340B cookie jar that patients are being squeezed out and left with crumbs. When legislators ask “is the intent of the program being met?”, these are the questions on their minds. Patients should have them on their minds as well.
For more information on the issues facing the 340B Program, you can access the Community Access National Network’s 340B Commission final report and reform recommendations here.
Sources:
Community Access National Network (February 2019). 340B DRUG DISCOUNT PROGRAM: The Issues Spurring Discussion, Stakeholder Stances and Possible Resolutions. 340B Commission Final Report. Retrieved online at https://docs.google.com/gview?url=http://www.tiicann.org/pdf-docs/2019_CANN_340B_Commission_Final-Report-v5_03-07-19.pdf&embedded=true
Fein, J. Adam (2020, May 19). Copay Maximizers Are Displacing Accumulators—But CMS Ignores How Payers Leverage Patient Support. Drug Channels. Retrieved online at https://www.drugchannels.net/2020/05/copay-maximizers-are-displacing.html
U.S Government Accountability Office (September 2011). DRUG PRICING: Manufacturer Discounts in the 340B Program Offer Benefits, but Federal Oversight Needs Improvement. GAO-11-836. Retrieved online at https://www.gao.gov/assets/gao-11-836.pdf
U.S. Government Accountability Office (June 2018). DRUG DISCOUNT PROGRAM: Federal Oversight of Compliance at 340B Contract Pharmacies Needs Improvement. GAO-18-480. Retrieved online at https://www.gao.gov/assets/gao-18-480.pdf
Office of the Federal Register (1996, August 23). 61 FR 43549 - Notice Regarding Section 602 of the Veterans Health Care Act of 1992; Contract Pharmacy Services. GovInfo.gov. Retrieved online at https://www.govinfo.gov/app/details/FR-1996-08-23/96-21485
Office of the Federal Register (2010, April 5). 75 FR 10272 - Notice Regarding 340B Drug Pricing Program-Contract Pharmacy Services. GovInfo.gov. Retrieved online at https://www.govinfo.gov/app/details/FR-2010-03-05/2010-4755
A Patient’s Guide to 340B: Why the Decline in Charity Care Matters to You
***This is the fourth report in a six-part series to educate patients about the 340B Drug Pricing Program***
A cornerstone argument in favor of the 340B Drug Pricing Program centers on so-called charity care rates of the participating Disproportionate Share Hospitals (DSH). Those covered entities, specifically DSHs, should be able to leverage their 340B dollars to extend care and out-patient medications to offset losses from uncompensated care. In the ideal, offsetting the costs associated with charity care to provide more care to low-income patients is noble and moral and just, and one society should support. The problem occurs when charity care is wrapped up or conflated with all “uncompensated, unreimbursed care” because a significant portion of uncompensated care is written off as bad debt, and that debt all too often gets reported to patients’ credit reports. Whereas charity care is care provided at no cost or debt to the patient. Moving forward, we must not confuse, conflate, or combine generalized uncompensated care with charity care.
The argument from the American Hospital Association is narrowly focused to present the rosiest picture, touting the totality of charity care provided by 340B DSH covered entities ($64 billion in of 2017, the latest available data as of the AHA’s statement). It ignores 340B participating hospitals have seen a steady decline in both charity care and uncompensated care, according to the Government Accountability Office’s 2018 report. The AHA’s own data reveals the same thing, despite exponential growth of the 340B program, largely attributed to hospitals and contract pharmacies. Unlike Federally Qualified Health Centers (FQHCs, a type of federal grantee entity in the 340B program), which are required provide care “regardless of ability to pay”, hospital systems, in large part, have a much more extensive debt collection program; they are not necessarily beholden to rules regarding debt collection practices. FQHCs, as an example, may be required to seek debt payments from internal billing specialists, but don’t generally have contracts to sell the bad debt to collections companies or report to credit bureaus. Furthermore, they are prohibited from doing so in certain circumstances.
While the Affordable Care Act (ACA) prohibited certain types of hospital-originated debt from being reported to credit bureaus, it doesn’t stop the hospital from selling the debt and then the collection company reporting the debt. Indeed, hospitals are notorious for reporting medical debt and sending bills to collections. If 340B dollars are meant to offset some of these expenses, with program growing about 23% per year, why does the Census Bureau report that about 20% of Americans are under some form of medical debt? Why has that medical debt grown from $81 billion in 2016 to $140 billion in 2019?
The ACA required non-profit hospitals to offer charity care programs, and the vast majority of hospitals across the country are non-profit hospitals. Adding insult to injury, that tax designation and the requirement to offer charity care hasn’t stopped these “non-profit” hospitals from chasing after low-income patients and further impoverishing them. A recent Kaiser Health News “An Arm and a Leg” podcast dove into just one state’s effort to tackle an epidemic of “non-profit” hospitals suing patients as a result of medical debt. The effort found a massive coalition of 60 entities, including a nurses’ union, and startling data supporting the need for Maryland’s now-passed “Medical debt Protection Act.”
Data included notation of almost 150,000 lawsuits against patients over the last 10 years, making almost $60 million from patients who would otherwise automatically qualify for charity care, and hospitals negotiating with for state funds to support charity care taking in $119 million than they actually gave out in charity care. And that’s just in one state. Indeed, according to information behind this report, Johns Hopkins – a 340B hospital – alone raked in $36 million more from this state-funded charity care support than they spent. While Maryland already had certain patient protections from these predatory practices on the books, too few patients knew about those protections and the state awarded these dollars without ever investigating the existing status of bad debt to charity care ratios. All the paper in the world written into the law is meaningless if affected people and corporations are not made to be transparent and held accountable.
Access to care, and freedom to access care, are two different things. Access to care being an open door, and freedom to access care is the freedom to walk through that door without fearing a dire financial consequence. While some special interests may argue the program is critical to hospitals extending access to care, their rhetoric lacks practical application when patients don’t have the freedom to access that care without fear of acquiring life-altering debt. The fear of medical debt keeps people away from seeking care. In fact, one of the most immediate and meaningful ways to tackle the country’s medical debt crisis would be for 340B covered entities to share the savings with patients. A patient’s medical debt reported to their personal credit file can, and does, perpetuate cycles of poverty; it is harming patients’ wealth, health, and overall well-being. If 340B dollars are supposed to be aimed at ensuring access to care, then concerns over medical financial toxicity shouldn’t be discounted.
Hospitals, in large part, though not universally, have seen a significant decrease in uncompensated care due to the ACA’s expansion of Medicaid. With more patients qualifying for Medicaid, meaning an ability for providers to be reimbursed where none previously existed, hospitals should be able to shift their uncompensated care burden from bad debt to patient financial assistance and charity care programs.
On the other end of decreases in charity care provided by 340B hospitals, are truly magnificent non-profit hospital chief executive officer compensation. In a 2019 hearing, CEOs admitted to having salaries in the millions of dollars per year range – that’s before bonuses. They also admitted to holding more money in reserves than they generally need in order to operate safely or not run the risk of running out of funding. Other instances of concern from this hearing include a hospital group using their 340B dollars to acquire a stand-alone oncology center. Typically, when these types of purchases are made, patients experience an increase in costs of care and sometimes experience a reduction in ability to access care due to an increase in patient load without subsequent staffing support or their provider’s office is physically moved as part of the consolidation effort, reducing a patient’s ability to physically get to and from office visits.
In addressing potential reforms that would benefit patient experiences, increase the sense of freedom patients feel to access care, and improve program efficacy, policy and law makers should both distinguish between generalized uncompensated care and charity care in annual financial reporting and 340B related audits and require a threshold of charity care for hospitals seeking to qualify for the 340B program. If hospitals are dissatisfied with their Medicare or Medicaid reimbursement rates and what that means for boosting their bottom line, they would do well to send their lobbyists after reimbursement dollars rather than disingenuously justifying their pilfer effort to rob 340B of its noble cause. Either way, it’s time these entities see requirements tied to their dollars, including rules around charging off debt against low-income patients.
For more information on the issues facing the 340B Program, you can access the Community Access National Network’s 340B Commission final report and reform recommendations here.
Sources:
American Hospital Association (September 2020). 340B Hospital Community Benefit Analysis. Retrieved online at https://www.aha.org/guidesreports/2020-09-10-340b-hospital-community-benefit-analysis.
American Hospital Association (December 2017). UNCOMPENSATED HOSPITAL CARE COST FACT SHEET. Retrieved online at https://www.aha.org/system/files/2018-01/2017-uncompensated-care-factsheet.pdf.
Bennett, N., Eggleston, J., Mykyta, L., & Sullivan, B. (2021, April 7). 19% of U.S. Households Could Not Afford to Pay for Medical Care Right Away. U.S. Census Bureau. Retrieved online at https://www.census.gov/library/stories/2021/04/who-had-medical-debt-in-united-states.html.
Community Access National Network (February 2019). 340B DRUG DISCOUNT PROGRAM: The Issues Spurring Discussion, Stakeholder Stances and Possible Resolutions. 340B Commission Final Report. Retrieved online at https://docs.google.com/gview?url=http://www.tiicann.org/pdf-docs/2019_CANN_340B_Commission_Final-Report-v5_03-07-19.pdf&embedded=true
Committee on Energy and Commerce, Subcommittee on Oversight & Investigations (2017, October 11). EXAMINING HOW COVERED ENTITIES UTILIZE THE 340B DRUG PRICING PROGRAM. U.S. House of Representatives. Retrieved online at https://www.govinfo.gov/content/pkg/CHRG-115hhrg27577/html/CHRG-115hhrg27577.htm.
Kliff, S. & Sanger-Katz, M. (2021, July 20). Americans’ Medical Debts Are Bigger Than Was Known, Totaling $140 Billion. The New York Times. Retrieved online at https://www.nytimes.com/2021/07/20/upshot/medical-debt-americans-medicaid.html.
U.S. Government Accountability Office (2018, June 18). Drug Discount Program: Characteristics of Hospitals Participating and Not Participating in the 340B Program. GAO-18-521R. Retrieved online at https://www.gao.gov/assets/gao-18-521r.pdf.
Weissmann, Dan (2021, October 7). PODCASTS – ‘An Arm and a Leg’: How One State Protects Patients From Hospital Lawsuits. Kaiser Health News. Retrieved online at https://khn.org/news/article/podcast-an-arm-and-a-leg-how-one-state-protects-patients-from-hospital-lawsuits/?utm_campaign=KHN%3A%20First%20Edition&utm_medium=email&_hsmi=168013149&_hsenc=p2ANqtz-_sXa4onkYlNPYAtzOv-Hn4b_y9OgQhBcwhnxYvtugSLuAXK1sYZ1_OWiArpRr29-YxGGpHZCZ9t4IhZVASXKpQZ-lS5A&utm_content=168013149&utm_source=hs_email.
A Patient’s Guide to 340B: Why Accountability Matters to You
***This is the third report in a six-part series to educate patients about the 340B Drug Pricing Program***
The word accountable is defined as “being required or expect to justify actions or decisions.” Accountability is often broadly discussed on a variety of levels about governmental and social issues, and the 340B Drug Pricing Program is certainly no exception. The 340B program exists to address the health care needs of a segment of society – social needs. As such, program accountability is of paramount importance since patient health depends on it.
Accountability in use of 340B dollars follows the benchmarks of transparency in reporting: federal grantees are required by contract to demonstrate patient benefit in use of program dollars and non-grantee covered entities are held to no such standard. Without fiscal transparency, non-grantee entities cannot be held accountable for their use of these revenues. The Health Resources Services Administration (HRSA) largely selects covered entities for audit based on a selection of “risk” characteristics. While some criticism of manufacturers is warranted in terms of accountability, manufacturers have only one statutory requirement. That requirement is to provide discounts or rebates on qualifying medications to covered entities. HRSA selects manufacturers for audit based on complaints from covered entities. Areas of complaint about manufacturers typically consist of overcharging a covered entity, not making a particular medication available, or not being transparent about the “ceiling price” of a drug.
To be fair, the statutory accountability requirements of 340B program are…limited and…vague. However, according to a 2020 report by the Government Accountability Office (GAO-20-108), the Health Resources and Services Administration (HRSA) severely lacks meaningful oversight, uniform assessment and request standards, and, as with many other reports, finds HRSA’s administration of the program to be largely inadequate.
As an example, GAO identified HRSA audits from 2017 and 2018 reviewed less than 10% of all non-governmental hospitals enrolled in the program. HRSA primarily relies upon hospitals to self-attest their eligibility. Of the selected hospitals participating in the GAO review, 18 submitted documents that would constitute a government contract – any description of a community program – and when HRSA found these instances, allowed the hospitals to avoid getting in trouble by acquiring contracts with retroactive applicability. All of that meaning, these hospitals in question did not experience any reprimand for failing to provide programming to low-income people but they got to enjoy the perks of unaccountable 340B dollars until they got caught. At the rate HRSA reviews these entities, it’s possible for a non-compliant or otherwise non-qualifying entity could go an entire decade soaking up dollars meant for patients in needs.
While HRSA’s annual 340B audits are primarily targeted toward covered entities, drug manufacturers are also audited to ensure they’re not charging covered entities more than they should be for 340B medications, to ensure drug manufacturers are not discriminating against covered entities, and make sure drug manufacturers are making sure their products are made available in compliance with the 340B program. Manufacturers represent about ten percent of annual audits, while covered entities represent about 90 percent and there are about 900 drug manufacturers participating in the program (dramatically less than covered entities). To be fair, GAO concluded HRSA also needed to provide clearer guidance to drug manufacturers regarding what qualifies as an acceptable distribution restriction due to anticipated or actual supply shortages and to provide specific guidance as to what constitutes “discrimination” of covered entity participants.
This issue of defining discrimination is developing and playing out in “real-time”. In May of 2021, HRSA announced notification letters sent to 6 manufacturers regarding their new policies requiring additional reporting from covered entities with contract pharmacies (as opposed to in-house pharmacies). HRSA’s interpretation of statutory language (“…shall…each covered entity…”) as non-discretionary on the part of manufacturers. In essence, if an entity is registered with HRSA for the program, a manufacturer is required by law to offer medications at ceiling price or below to that entity, regardless of any potential for a covered entity to use program dollars outside of the intent of the program. While skepticism of non-grantee use of these dollars may be warranted due to lack of transparency in use of these dollars, diversion, or duplicate discount concerns, given that federal grantees are already required to report use of these dollars to their federal funders, a more narrowly tailored policy directed exclusively at non-grantee covered entities would be more appropriate to address the interest needs of manufacturers, the public, and program stability. However, given HRSA’s interpretation of the statutory language, even such a proposal might run the risk of rubbing regulators wrong. At the time of this writing, at least one of the manufacturers has sued the Department of Health and Human Services to prevent any monetary penalties related to these letters from being imposed. A judge has dismissed the government’s opposition to the suit in June of 2021. And on September 22, 2021 HRSA issued letters to the manufacturers in question, stating the issue had been referred to the Office of the Inspector General.
Lack of transparency means less accountability. Patients are better served when 340B-related dollars remain within the same geographic area they were generated by the covered entities. After all, if serving low-income patients means serving community and getting usable revenue required to be used on low-income patients, those dollars should be put back into the same community in which they were generated, right? But covered entities with large networks and multiple covered entity sites aren’t required to show those revenues are reinvested in the same area they were generated. For instance, monies made off the health and illness of an Atlanta community should not be spent to buy up profit generating imaging machines in a well-to-do suburban area outside of Los Angeles. But, without both transparency and accountability, 340B dollars can easily become a slush fund of revenues for any industrious non-grantee covered entity.
Indeed, many large contract pharmacies offer software programs to covered entities as a measure of their own “transparency” with internal reporting but the real goal is to show the covered entities “here’s how you can make more 340B dollars” – but at a cost of providing the service and without uniform assessment metrics. That means the contract pharmacy can tilt the experience of a patient by applying pressure to the covered entities very subtly through software programs telling the provider, “You can make more money off this patient by prescribing…”. Advocates have very good reason to be suspicious of contract pharmacies associated with (or even owning) pharmacy benefit managers who, then, can very easily provide programming that targets their profits over ensuring rebate dollars make it back to a patient.
Statutory clarification could greatly benefit the intended purpose of the 340B program – ensuring low-income patients get the care they need by taking a few, simple steps, specifying reporting requirements that mirror existing transparency and accountability found among grantees. Additional oversight is needed in numerous areas, all designed to further benefit patient access to care and medications. Among them, non-grantee entities should be required to report how 340B dollars are being used, by which payer source a claim is generated, how much charity care a non-grantee entity provides, and how much revenue is generated from pharmacy sales (and how much is generated from 340B sales). Patients might not understand the nuances behind the program complexities, but they do understand when they cannot access the care they need and deserve. If the purpose of the 340B program is to expand access to care and medications, then why not go that extra mile?
Congress could go a great deal further to ensure these billions of dollars serve patients, rather than the interests of shareholders in private hospital systems or propping-up senior management compensation packages, or other non-medically-related expenses. Congress could also opt to provide for additional minimum requirements in order to qualify as a covered entity – especially with regard to private hospitals providing a certain percentage of charity care.
For more information on the issues facing the 340B Program, you can access the Community Access National Network’s 340B Commission final report and reform recommendations here.
Sources:
Community Access National Network (February 2019). 340B DRUG DISCOUNT PROGRAM: The Issues Spurring Discussion, Stakeholder Stances and Possible Resolutions. 340B Commission Final Report. Retrieved online at https://docs.google.com/gview?url=http://www.tiicann.org/pdf-docs/2019_CANN_340B_Commission_Final-Report-v5_03-07-19.pdf&embedded=true
Health Resources Services Administration (September 2021). 340B Drug Pricing Program – Program Integrity. HRSA Correspondence to Stakeholders 2021. U.S. Department of Health & Human Resources. Retrieved online at https://www.hrsa.gov/opa/program-integrity/index.html
Lagasse, Jeff (June 2021). Judge dismisses HHS Challenge of AstraZeneca's 340B Contract Pharmacies Lawsuit. HealthCare Finance. Retrieved online at https://www.healthcarefinancenews.com/news/judge-dismisses-hhs-challenge-astrazenecas-340b-contract-pharmacies-lawsuit
U.S. Government Accountability Office (December 2019). 340B DRUG DISCOUNT PROGRAM: Increased Oversight Needed to Ensure Nongovernmental Hospitals Meet Eligibility Requirements. GAO-20-108. Retrieved online at https://www.gao.gov/assets/gao-20-108.pdf
A Patient’s Guide to 340B: Why Transparency Matters to You
***This is the second report in a six-part series to educate patients about the 340B Drug Pricing Program***
All public-private partnerships require transparency to instill confidence in program function, private business operations, and government accountability. Transparency is an essential part of the equation; it brings us more accountability and more effective programs. It helps to identify areas of improvement in operations or enforcement, as well as limiting waste, fraud, and abuse. The 340B Drug Discount Program is no exception because transparency ensures investments into patient access to medications for critically vulnerable populations are reaching patients. Transparency – in every programmatic aspect – serves the public interest and is, frankly, just good government. It builds confidence in the efficacy of the program and good will of the participating entities.
In general, under the 340B program, those entities receiving federal grant funding – known as “federal grantees” – under other programs (i.e., federally qualified health centers, Ryan White HIV/AIDS clinics, hemophilia centers, and others) receive a great deal over oversight on how they use their discounts and rebates from 340B, though that oversight comes as part of their fiscal reporting under those other programs. For non-grantee covered entities, oversight is primarily dependent on audits and self-attestation of compliance and corrections to issues. With non-grantee covered entities lacking dedicated oversight like federal grantees, there’s a lack of transparency in how those entities qualify under the program and how those entities are using 340B-generated revenues to benefit low-income patients.
Regardless of program, dollars meant to serve low-income patients are often scarce. As such, patients lose when the investments needed to support and expand services for vulnerable populations are directed elsewhere (outside of the community those dollars originated from or for-profit building purposes). Patients lose out on funding support that keeps programs stable, ensures access to critical health programs nearest to them, and ultimately threatens to destabilize a program relied upon by the federal government and community stakeholders to keep clinic and hospital doors open.
At the inception of the 340B program, legislation such as the Patient Protection and Affordable Care Act did not exist, and only 29 million people nationwide were enrolled in Medicaid. Fast forward to 2018, Medicaid rolls had grown to 72 million people – meaning in all but the hold-out “non-expansion states” nearly any hospital in the country might qualify as a “disproportionate share hospital” – a situation 340B never considered at inception. The development and growth of the program was analyzed in a 2018 report issued by the U.S. House of Representatives’ Committee on Energy & Commerce.
According to a Government Accountability Office report (GAO-21-107) about 80% of current covered entities are federal grantees and 20% of covered entities are hospitals. However, many of these entities, especially hospitals operate multiple sites – not all entities are created equal in terms of generating program revenue. Of the approximate 37,500 covered entity sites participating in the program, about 75% of those sites are hospital affiliated with hospitals, not federal grantees. Hospitals are able to qualify specifically because of the low threshold of “disproportionate share” of low-income patients who can now afford to seek care thanks to Medicaid expansion – even if the hospital entity is generally well off enough to not actually need those dollars in order to provide care. In order to better understand how these changes have impacted growth and qualification of the program, “disproportionate share” may not be the best formula to ensure 340B dollars are helping those who need it most. Particularly, given the decreasing share of charity care certain hospital entities have offered over the years, evaluating charity care percentages and qualifying patients by income and payer type (self-pay, Medicaid, private insurance, etc.) may be more accurate in ensuring entities are actually serving low-income communities.
To be clear, “charity care” is a specific type of “uncompensated care” – or when patients receive care but can’t pay their bills. Unlike other types of uncompensated care, whereby providers may send a patient’s bill to a collections company, charity care releases the patient from a portion or all of their financial responsibility. Typically, charity care is limited to those who have to choose between putting food on their table and seeking preventative care like mammograms or having to decide in what life-saving neonatal care a family might need. Given the intersection of race and poverty in this country, charity care is a critical, even if anecdotal measure of how much a hospital is invested in their local community and combating community health disparities like pregnancy-related mortality.
The 340B program’s statutory language is largely silent on how these revenues dollars may be spent and because of that, there’s little to ensure these dollars are actually going to benefit patients instead of hospital networks or pad executive pay. Patient advocates have long crowed about the need for non-grantee covered entities to meet the same transparency requirements federal grantees are required to meet. Indeed, one of the biggest challenges facing the 340B program is better understanding how these dollars are spent. Now, typically, where statute is vague, government agencies tasked with managing programs have the regulatory power to make rules and the man power to enforce them. That’s just not the case with 340B and the Health Resources and Services Administration (HRSA) has repeatedly stated a lack of surety in its ability to regulate beyond guidance and frequently cited an inability to expand auditing capacity due to lack of funding. So much so that President Biden included $17 million in his budget request to strengthen and expand oversight of the program specifically in terms of auditing how 340B revenues are generated and spent among on-grantee covered entities.
Given the program’s growth, there’s reason and need to further clarify the intent of the program, cemented into unambiguous statutory language to reflect the country’s health care landscape of today and ensure the revenues generated are actually helping patients and not padding executive pockets. In our next blog, we’ll discuss the accountability processes currently in play for covered entities and manufacturers and the glaring holes in that part of the oversight “net”.
For more information on the issues facing the 340B Program, you can access the Community Access National Network’s 340B Commission final report and reform recommendations here 2018 report.
Sources:
Centers for Disease Control & Prevention (2020, February 4). Infographic: Racial/Ethnic Disparities in Pregnancy-Related Deaths — United States, 2007–2016. U.S. Department of Health & Human Services. Retrieved online at https://www.cdc.gov/reproductivehealth/maternal-mortality/disparities-pregnancy-related-deaths/infographic.html
Community Access National Network (February 2019). 340B DRUG DISCOUNT PROGRAM: The Issues Spurring Discussion, Stakeholder Stances and Possible Resolutions. 340B Commission Final Report. Retrieved online at https://docs.google.com/gview?url=http://www.tiicann.org/pdf-docs/2019_CANN_340B_Commission_Final-Report-v5_03-07-19.pdf&embedded=true
House Committee on Energy & Commerce (2018). Review of the 340B Drug Pricing Program. U.S. House of Representatives. Retrieved online at https://republicans-energycommerce.house.gov/wp-content/uploads/2018/01/20180110Review_of_the_340B_Drug_Pricing_Program.pdf
King, Robert (2021, June 1). From 340B to a public option: 4 healthcare items you may have missed in Biden's budget. Fierce Healthcare. Retrieved online at https://www.fiercehealthcare.com/hospitals/from-340b-to-a-public-option-4-healthcare-items-you-may-have-missed-biden-s-budget
U.S. Government Accountability Office (December 2020). DRUG PRICING PROGRAM: HHS Uses Multiple Mechanisms to Help Ensure Compliance with 340B Requirements. GAO-21-107. Retrieved online at https://www.gao.gov/assets/gao-21-107.pdf
A Patient’s Guide to 340B: Why the Program Matters to You
***This is the first report in a six-part series to educate patients about the 340B Drug Pricing Program***
In 1992, Congress struck a deal with pharmaceutical manufacturers to expand access to care and medication for more patients: If pharmaceutical manufacturers wanted to be included in Medicaid’s coverage, they’d have to offer their products to outpatient entities serving low-income patients at a discount. The idea was brilliantly simple; drug manufacturers could have a guaranteed income from participation in the Medicaid program, and “covered entities” could have guaranteed access to discounted medications. Congress set-up a payment system by way of rebates, affording healthcare providers a way to fund much-needed care to patients who could not otherwise afford it.
This payment program is little known but, now it is significantly large. It is the 340B Drug Pricing Program.
“At the inception [of the 340B program], these entities [Hemophilia Treatment Centers (caring for all patients with both bleeding and clotting disorders), Ryan White Clinics and FQHCs were specifically identified] were the prime targets to benefit from the three major goals of the initial PHS pricing program: first, that pharmaceutical products would be purchased at markedly reduced 340B pricing; secondly, the discounts would be passed on to the payors and finally that a small, reasonable, percentage would go to the entity itself, to sustain Covered Entities to care and expand diagnostic and clinical services.”
– Dr. Diane Nugent, National Commission on 340B (2018)
Initially, covered entities were exceptionally restricted, including but not limited to federally qualified heath centers (FQHCs), Ryan White HIV/AIDS clinics, hemophilia treatment centers, and only one category of hospitals, so-called “disproportionate share hospitals” (DSH). DSH is a hospital entity that provides a “disproportionate” number of low-income patients, evaluated quarterly and calculated through a formula dictated by statute. Of these entities, those receiving federal grant dollars under any number of federally funded programs are called “federal grantees”.
Federal grantees are required by statutory language to certain transparency in how they spend their 340B-related revenue. In trade, participating drug manufacturers are also required to be transparent in their contributions to the program.
In addition, federal grantees are required to be transparent and accountable regarding their 340B-generated dollars by their federal grants, not by the statutory language of the 340B program. That means every dollar a federal grantee generates is held accountable to serving the needs of low-income patients. How these dollars may be used from grantee to grantee may look a little different but they’re still required to fit within the guardrails of the grant and, for many federal grantees, the most direct way of achieving this goal is sharing the savings with patients at the pharmacy counter. By its very nature, 340B’s purpose is to reduce the amount of tax dollars spent on these grants by providing an avenue of program revenue, and thus support existing efforts to provide care for the most vulnerable.
Over the years, covered entities have expanded to include contract pharmacies, family planning centers, children’s hospitals, critical access hospitals, rural referral centers, freestanding cancer centers, and sole community hospitals. From 1992 until about 2001, participation in the program by covered entities was fairly static – it didn’t grow or change in any massive quantity. After 2001, covered entities able to access the 340B program began to grow at an exceedingly fast pace, with even more growth among “covered entity sites” and the greatest amount of growth among contracted pharmacies. This was reflected in 340B sales, as well. According to the Drug Channels Institute, 340B purchases grew from about $2.4 billion in 2005 to more than $38 billion in 2020.
In general, 340B-related income looks like an insurer reimbursing the cost of a medication for a patient to a covered entity, a pharmacy filling the medication at the rebated cost with addition of a minor dispensing fee, and the covered entity keeping the excess as savings. Covered entities are allowed spend those excess funds in particular ways which qualify as “expanding access” to medication or care. For entities applying those funds directly to outpatient medications, this is known as “following the patient” or “sharing the savings”. Other uses may include anything that directly impacts access to or quality of care for low-income patients. Notable examples may include technology upgrades to be in-line with patient security and best practices in extending scarce human resources (i.e. how efficient care can be delivered to patients), acquiring new care technology to provide care not previously available (i.e. imaging and x-ray machines), and infrastructure like mobile medical units in order to bring care to patients rather than bringing patients to care or opening new locations in order to be more accessible to their served communities. 340B prohibits covered entities on double dipping on discounts or applying rebate dollars to inpatient medications or to a particular patient that does not qualify as low-income (“diversion”).
That patient getting their share of the savings makes a great deal of sense. Indeed, a Government Accountability Office report (GAO-18-480) of selected covered entities stated of 55 interviewees, 30 reported providing low-income, uninsured patients on 340B dispensed medications and all “30 covered entities providing patients with discounts reported providing discounts on the drug price for some or all 340B drugs dispensed at contract pharmacies. Federal grantees were more likely than hospitals to provide such discounts and to provide them at all contract pharmacies.” Patients realize the savings of the rebate program immediately. Benefits of the program which may be less recognizable to patients for a similar report from 2011 (GAO-11-836) included funding a non-revenue-generating case management program, patient and family education programs similar to guidance pharmacists provide on medication interactions, and transportation to and from care appointments. All of which are critically necessary in terms of creating a safety net of accessible care for vulnerable communities and patients.
For more information on the issues facing the 340B Drug Pricing Program, you can access the Community Access National Network’s 340B Commission final report and reform recommendations here 340B Drug Pricing Program.
Sources:
Community Access National Network (February 2019). 340B DRUG DISCOUNT PROGRAM: The Issues Spurring Discussion, Stakeholder Stances and Possible Resolutions. 340B Commission Final Report. Retrieved online at https://docs.google.com/gview?url=http://www.tiicann.org/pdf-docs/2019_CANN_340B_Commission_Final-Report-v5_03-07-19.pdf&embedded=true
Hasan, Shiraz, and Sofi Peterson (2021, April 16). 340B Drug Discount Program Growth Drivers. IQVIA. Retrieved online at https://www.iqvia.com/locations/united-states/blogs/2021/04/340b-drug-discount-program-growth-drivers
Health Resources & Services Administration (2021). Sec. 340B PUBLIC HEALTH SERVICE ACT. U.S. Department of Health & Human Services. Retrieved online at https://www.hrsa.gov/sites/default/files/opa/programrequirements/phsactsection340b.pdf
U.S. Government Accountability Office (2011, September). DRUG PRICING: Manufacturer Discounts in the 340B Program Offer Benefits, but Federal Oversight Needs Improvement. GAO-11-836. Retrieved online at https://www.gao.gov/assets/gao-11-836.pdf
U.S. Government Accountability Office (2018, June). DRUG DISCOUNT PROGRAM: Federal Oversight of Compliance at 340B Contract Pharmacies Needs Improvement. GAO-18-480. Retrieved online at https://www.gao.gov/assets/gao-18-480.pdf
Where Public and Private Payers Fail, Patient Assistance Programs Step In
During Community Access National Network’s Co-Infection Annual Monitoring Report, hosted during this year’s SYNC Conference, in reviewing Hepatitis C (HCV) treatment coverages offered by state Medicaid and State AIDS Drug Assistance Programs (ADAPs), I stated “ADAPs play a critical role even in Medicaid expansion states. With the cost of medications for both HIV and HCV, even making just about the Medicaid threshold can create a catastrophic gap in patient financial stability. Given the distinct exposure risks shared for both disease states, ADAP advocates should consider the advantages of their programs to fill this need.” And…
Earlier this year, I wrote a guest blog for ADAP Advocacy Association regarding the funding situation for Georgia’s AIDS Drug Assistance Program in light of shortfalls due to COVID-19. While Georgia has since enjoyed the benefit of astounding state advocates’ work, a hole in the program remains. At the bottom of Georgia’s most recent ADAP formulary a notice reads as follows: “Georgia ADAP Hepatitis C Program is currently on HOLD until future funding is available. Please utilize Patient Assistance Programs (PAP’s) for Hepatitis C medications.” Georgia wasn’t the only state to consider ceasing coverage of HCV medications, Texas did as well, though Texas has since added a single direct acting agent back into their ADAP formulary. In discussing the state’s funding shortfalls earlier this year, state representatives mentioned to providers referral to patient assistance programs in order to meet patients’ needs.
The summation here is publicly funded programs are not always aptly designed to meet the needs of vulnerable populations. Indeed, Harvard’s Center for Health Law and Policy outlines barriers to care instituted by public payers, like ADAPs and Medicaid programs, in an annual report. These barriers largely mirror the barriers to care instituted by private payers, wherein private payers argue these are necessary “cost containment” measures (otherwise referred to as “utilization management”). However, for patients, these “cost containment” measures largely amount to “care containment” measures and, frankly, do little more than push patients out of care through administrative burdens, commonly manifested as lengthy appeals processes or requirements for sobriety.
When public and private payers fail patients by refusing coverage of HCV treatments, they also fail our public health goals, perpetuating preventable illness and death. In the space where payers deny coverage and where people cannot afford coverage due to premiums or deductibles or being too high or where patients simply can’t afford insurance coverage and can’t access public programs, patient assistance programs step in. Patient assistance programs may be run and funded by medication manufacturers or by community-based funding programs and navigation information tools.
CANN’s quarterly Co-Infection Watch monitors HCV specific patient assistance programs for status and limits, in order to make identifying resources easy for patients and advocates reviewing the report.
While these tools are excellent tools of last-resort, they are limited and cannot be used as a substitute to sufficient public funding, regulation or legislation ensuring non-discriminatory plan designs, and adequate patient protections. Public payers have come a long way in abandoning caustic programmatic barriers for patients to jump through in order to access care and medication and private players have much farther to go. Regardless of circumstance, neglecting this critical medical intersection by way of foregoing HCV medication coverage is a public health and public payer program failure. All it does is kick a snowball down a hill. We’re already set to fail meeting our 2030 goals toward HCV elimination. We must do better to ensure this problem, at the very least, doesn’t grow.
HIV & Covid-19: A Story of Concurrent Pandemics
On September 20th, Johns Hopkins’ COVID data tracker totaled the “confirmed” (note: not “official”) number of deaths from COVID-19 in the United States to surpass 675,000 – or the estimated number of deaths in the US due to the 1918-1919 H1N1 influenza pandemic (colloquially called the “Spanish flu” because Spanish media were more willing to discuss the pandemic than most other countries). Forbes, STAT, and other large news outlets ran headlines like “Covid-19 overtakes 1918 Spanish flu as deadliest disease in American history” or included statements in their articles like “It was the most deadly pandemic in U.S. history until Monday, when confirmed coronavirus deaths overtook the death toll for the Spanish Flu.”
Which, as Peter Staley pointed out, isn’t factually accurate.
Image: Twitter.com - @peterstaley (Sep 20, 2021) “Um, HIV/AIDS? 700,000 U.S. deaths (and counting), according to the http://HIV.gov https://hiv.gov/federal-response/ending-the-hiv-epidemic/overview”
Staley would quickly admit COVID-19 would or already has likely overcome the death toll of HIV in the United States. While I agree with this analysis, I would add “for now”.
The very nature of HIV has made finding a “cure” or vaccine for the virus an oft sought after “holy grail” in pharmaceutical development. While that grail may have been snatched away by the attention COVID-19 is justly generating, this isn’t the first concurrent pandemic HIV has run alongside. The Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) both refer to the H1N1 influenza outbreak of the 2009-2010 flue season a “pandemic”. The problem of course isn’t just how deadly COVID-19 is, its’ how botched the domestic and global responses have been to the disease.
Viruses, after all, are opportunistic. They have a singular purpose: reproduce. As such, viruses thrive in environments – ecosystems, if you will – that are sorely neglected, lack coordinated responses, and are largely inequitable. But we knew that. We’ve known that with regard to global and domestic health disparities data for decades. As with personal health, emerging, urgent issues in public health reduce our capacity to address existing issues effectively.
As I mentioned in previous blogs, and has been recently noted by the Global Fund, COVID-19 has drastically reduced the efficacy of existing HIV, HCV, STI, and SUD programs. Even still, Global Fund’s report proves a rather interesting point – when meeting the demands of advocates for programs to provide patients with multi-month supplies of medications, meeting people in their own neighborhoods rather than in clinics, and providing at-home testing kits, communities can be activated in care at an exceptional level. Despite the COVID-19 pandemic raging, the needs of the HIV pandemic didn’t stop. And while meeting those needs faltered some (with 4.5% fewer mothers receiving vertical transmission prevention medications, an 11% drop in prevention programming, and a 22% reduction in testing services), in some areas meeting those needs thrived. Global Fund’s report found South Africa was able to increase the number of people receiving antiretroviral therapies by more than three times the baseline, even while fighting on two fronts.
Dr. Sioban Crowley, Head of HIV at the Global Fund, pointed out these program designs are not exclusive to HIV, “If we can keep 21.9 million people on treatment, we can probably deliver them a COVID test and a vaccine.”
Indeed, with the United States’ (and the world’s) response relying heavily on expertise gained in the fight against HIV, one can reasonably ask “If we know how to beat this, why aren’t we…just doing that?”
“That” being what advocates have long asked for: a more dedicated, equitable landscape and adequate support of our public health systems. As with COVID-19, a vaccine won’t “cure” us of HIV if the rest of the world cannot access it. As with HIV, if preventative services, adequate testing, and necessary education are not readily made available to people where they are, we will continue to fail in both fights. If we don’t wish to repeat the losses we’ve already experienced in the fight against HIV, then we cannot keep making the same mistakes of kicking the costs of these investments down the road and maybe, eventually “getting to it”.
As has been said many times through the latest pandemic, “the best time to do the right thing was yesterday. The next best time to do the right thing is today.” It’s time for us to do the right thing and stop allowing backbone public health programs to fall by the wayside in the face of the next emergency. Today, for the next few years, it’s COVID. We don’t need to “wait” for that to end. There’s two pandemics occurring, it’s time we act like it.
Coverages & Pitfalls: Pandemic-Related Health Care Expansion
On September 17th, the Centers for Medicare and Medicaid Services (CMS) announced its first complete rule on health care marketplaces (federal and state – HealthCare.gov and SBEs), certain expectations of insurers participating in the health care marketplace, and a slate of other issues. This final rule serves as a regulatory tool amid a raft of information the federal government has provided regarding health insurance coverage during the pandemic.
Portions of the rule were fairly well expected (extending the annual open enrollment period from the slimmed down 45 days the previous administration imposed and revamping the “navigators” program), while other portions sought to more narrowly address – read “stop” or “reverse” – changes from the previous administration (specifically those introduced in 2018 and others introduced on January 19, 2021). The rule also aims to address some pressing concerns from legislators and health care access advocates about affordability of insurance as the economic future of the country remains unstable with the COVID-19 pandemic still wreaking havoc on much of the country.
One September 14th and 15th, the Census Bureau and the Department of Health and Human Services (HHS), respectively, issued data related to insurance coverage among residents of the United States in 2020 and 2021. The Census data diverged slightly from the HHS data in that the Census data did not show an increase in the number of people enrolled in Medicaid from 2018 to 2020, whereas previously released CMS data had shown a substantial increase (15.6% or about 10.5 million people) in Medicaid and Children’s Health Insurance Program (CHIP) enrollment from February 2020 through March 2021. A report from the Urban Institute cites potential for the remainder of 2021 to net an additional 17 million people enrolled into the safety net health insurance programs. All of this coincides with the Census data showing the uninsured rate was near static from 2018 to 2020 (8.6% compared to 8.5%).
This is a pretty remarkable comparison, given the pandemic’s effects on the country’s economy. The Census does cite a reported drop in employment related coverage during 2020, with the highest rate of employer sponsored coverage drop occurring among those employed less than full-time. Indeed, the data shows the largest drop in employer sponsored coverage occurred for those at the lowest end of the compensation scale.
Part of why tying together the employment data of the Census and the Medicaid and CHIP enrollment growth data together is to better understand the severe risks to vulnerable people and families as the seeks to wrap up the public health emergency declarations (likely sometime next year). While the administration’s payment rule highlights efforts to keep afloat lower- and middle-income families afloat and insured, the same can’t be said of the lowest-income earners. While the Biden Administration has extended the period for states to return to enrollment recertifications for Medicaid, federal matching funds (a boosted benefit to states during the pandemic) are expected to end on a similar timeframe, giving states some added financial motivation to move through disenrolling current recipients quickly, rather than in a staggered fashion.
While we outlined steps state Medicaid programs and safety net providers could take at the wrap up of the PHE in a blog earlier this month, the Biden Administration must seek even more moves than currently planned, to ensure “back to normal” doesn’t amount to “back to broke” for low-income families across the country.
Veterans Linkage to Care: Perspectives on HIV, Viral Hepatitis, Opioids & Mental Health
Approximately 8 percent of the U.S. population are Veterans, numbering over 18 million Americans with most of them being males and older than nonveterans. But those demographics will change in the coming years, with significant increases in ranks among women and minorities. As a society, we tend to view these men and women formerly in uniform as larger than life figures capable of overcoming almost any odds. The reality, however, is there are numerous ongoing public health challenges faced by Veterans in this country once discharged from the military – among them HIV, Hepatitis C, opioid dependence, and mental health conditions. As a society, don’t we owe it to them to provide the most timely, appropriate linkages to care and treatment?
In 2019, there were 31,000 Veterans living with HIV seeking care within the Veterans Affairs (VA) healthcare system. Additionally, 3.4 million Veterans were eligible for HIV screening. Navigating the VA is challenging enough for our Veterans, but imagine doing so after first being diagnosed with a lifelong, chronic illness like HIV/AIDS? Although no longer a death sentence, Veterans need to learn how to steer living with HIV in what seems like a battlefield of complex bureaucratic systems, simply to start their care and treatment. For Veterans, staying connected to appropriate levels of care continues to be vital for many reasons.
For example, pulmonary hypertension – a blood pressure condition that affects the lungs and heart – is higher among Veterans living with HIV than in veterans who don’t have an HIV-positive diagnosis. What adds an extra level of concern is that Veterans with a CD4 count below 200 are also at higher risk of pulmonary hypertension, including Veterans who have viral loads higher than 500 copies per mL. Pulmonary hypertension within itself is a rare condition but that is exactly the reason why Veterans needs to remain linked to their healthcare providers. Some healthcare providers may not be actively probing for rare conditions like pulmonary hypertension and thus the condition and its possible progression will go largely undiagnosed. This further places into perspective the wide net needed in appropriate, timely HIV care and treatment that goes beyond taking antiretroviral (ARV) medication to achieve viral suppression.
Advances in HIV medicine – namely the introduction of the highly active antiretroviral treatment in 1996 – changed how people can live their lives after an HIV-diagnosis. Whereas people living with HIV who are virally suppressed have the same life expectancy as their non-positive counterparts, they’re also prone to age-related conditions and other co-morbidities, such as the previously discussed pulmonary hypertension. What this also means is that living longer, fuller lives also opens-up the door to emotional distresses.
Newly enlisted service members cycle through intense emotions when shifting from civilian life to the demands of military culture. Post discharge, Veterans can find themselves yet again cycling through acute reactions as they struggle to respond back into the reintegration of the everyday family and civilian life. As a result, studies have shown that incidences of ischemic stroke, the most common type, is more prevalent in Veterans who are HIV-positive dually diagnosed with depression in comparison to Veterans who don’t have a positive diagnosis without depression. This is significant because a common psychological effect of depression is isolation. Without proper linkages to care, so many human pathways of connectivity can begin to become severed. Positive behaviors and patterns begin to change, and this is a dangerous mental state to be in not only as a Veteran struggling with civilian life, but also maintaining the healthy and consistent level of care and treatment that is needed for Veterans living with HIV. It opens the door to poor medication adherence, decreased social networks, and increased likelihood of substance use disorder. These landmines are crucial markers to ensure Veterans living with HIV are kept engaged in their treatment plans. Likewise, all clinicians need to do the same by remembering to evolve with their clients to continue providing them with the services that they need and deserve.
Another silent threat facing both Veterans and nonveterans alike is Hepatitis C (HCV). The Centers for Disease Control & Prevention (CDC) estimates there are nearly 2.4 Americans living with HCV. It continues to remain a public threat to the general population, but it particularly relevant to address how the silent epidemic is impacting Veterans.
If left untreated, HCV can be fatal because it can lead to cirrhosis of the liver. Veterans experience chronic HCV at three times the rate of the general population, with 174,000 Veterans in active care within the VA system. So, what factors need to be considered by Veterans seeking testing and treatment options for HCV? After all, modern medicine continuously changes the landscape of the available medical treatment options, and the constant reevaluation can be overwhelming. Fortunately, newer HCV therapies have made it a little easier. A qualitative analysis of 29 Veterans who were looking into HCV treatment, 35 total factors were of interest were identified. Of this set of 35 attributes, the top reported were treatment efficacy, physical side effects, new antiviral drugs in the pipeline, liver condition, and psychological side effects.
While the report’s findings aren’t necessarily surprising, how they structured their analysis is important. The Veterans in this study were placed in one of three categories that identified their personal stage of change – which were contemplating treatment, recently declined treatment, and recently initiated treatment. Successful linkages to care involve acknowledging where clients are in the process because it helps to identify and structure a patient centered treatment plan. What is important to remember is that each stage of change is shaped by the personal lived experiences clients are currently experiencing. Some of these subfactors are important social systems that they interchangeably occupy like family, friends, work, religion, and perhaps other various community engagements. All of which can greatly affect the decision-making process when considering treatment. Clinicians across the board need to have a clear picture as to what their client’s value and integrate those value systems into the appropriate levels of care to maximize the effectiveness of their treatment.
This same study uncovered another point of interest that is worth mentioning. When it came to gender, 50% of women compared to 14% of men, reported having concerns with social attributes like stress on partnerships and stigma associated with a disease. Additionally, women also reported concerns about maintaining their privacy within the systems that they occupy. In some ways these results are not surprising given the long history of women being undervalued and overexposed within society. That said, what this does highlight is how the concept and execution of healthcare needs the integration of a vast interpersonal team across a diverse and all-encompassing platform that has the capability to target these pockets of influence.
Healthcare disparities, unfortunately, exist across a wide spectrum within our medical framework and the VA isn’t immune from it. For minority Veterans with hepatitis C, seeking treatment are faced with unique barriers. For example, an HCV-diagnosis is four times more likely among minority Veterans compared to the general population. The VA’s Office of Health Equity (OHE) has done some great work in eliminating health disparities among minority Veterans with HCV, including testing. Testing is made available to all Veterans who are enrolled in the VA; they have treated more than 123,000 Veterans, and successfully cured more than 105,000 Veterans. The VA’s vigorous approach to its mission has been met with great results as race and ethnicity proportions are being treated equally with no population higher than the other. Effective strategies like video telehealth, the use of nonphysician providers, and electronic data tools for timely patient tracking and outreach have allowed the VA to expand their services to better address gaps in care. Work like this is needed across VA systems and local communities to minimize the gaps that are all too often seen in minority groups especially when there are 50,000 Veterans who are undiagnosed for HCV.
Any discussion about linkages to care needs to address the risk association between Hepatitis C and opioids. Since 2010, there have been correlating spikes in both. According to the CDC, HCV cases have nearly tripled between 2010-2015, and during this time the growing use of opioids exploded thanks to OxyContin, Vicodin, morphine, and fentanyl.
Like the general population, substance use disorder can be an inherited experience for Veterans, sometimes exacerbated by the effects of military culture. As a result, 1.3 million Veterans experience levels of substance use disorder. A study by the VA Health System in 2011 indicated that Veterans, when compared to the general population, are twice as likely to experience death from an opioid overdose incident. The biggest leading factor in this is prescription opioid medication and it continues to increase. In 2005, 4 percent of service members reported misusing their prescription medication. Three years later, 11 percent of service members reported the same misuse. The challenge here is that military culture demands a high level of sacrifice, which often comes with potential risk factors like bodily injuries and exposure to traumatic events. Both can be a slippery slope. Physical injury begins to be a major factor almost immediately after enlisting. Service members are pushed daily to exercise and ushered through a series of combat drills that will no doubt include heavy equipment. The body has a great ability to adapt and strengthen itself but like anything else, it has its limits. If this sets the stage for a revolving door of service members in physical pain, the natural course of action would be to provide medication to offset these symptoms. And just like that, accessibility without effective evaluations become the gateway to opioid substance use.
In the same fashion, traumatic events can leave service members feeling disconnected from where they’d like to be both emotionally and physically. In military culture, perception of strength is reality and as such, seeking services for mental health is often challenging for servicemembers as they don’t want to appear weak, so they suffer in silence. But that is exactly the reason why work is needed to change this outcome. Military culture to a very large degree is unwavering. It needs to build soldiers and do that; it needs to condition enlistees. However, it would be beneficial if clinicians and doctors within military culture to incorporate better systems of evaluation when it comes to pain management. This would also need to extend into the various VA systems that service members have access to. Relationships and bonds are obviously built within military culture and their importance may be of great benefit when combating the negative effects of stigma associated with mental health trauma. Community programs can be fostered and guided by various ranking officers to establish a sub community where conversations of real-life experiences demonstrate that a soldier of any rank can be supported by the comrades and communities that they protect.
But accessibility is a two-way street. Clients should have the ability to gain access to healthcare to receive treatment for various medical concerns. Clinicians or outreach programs should be able to have access to community members that need a particular public health service. Syringe services programs (SSP) introduced in the 1980s, have been adopted by the VA system to reduce the harm for Veterans who inject drugs . Veterans who utilize SSP’s can receive substance use and mental health services with the VA including additional services through an SSP program like vaccinations and naloxone, which helps to prevent an accidental overdose. Veterans benefit from community-based programs like this even with the controversies that the program may still carry since its inception. This program has been proven effective in reducing transmission of disease like HIV and Hepatitis C. While this program isn’t stopping the use intravenous drug use, it does open the door for Veterans who may be in a place mentally to accept help. Programs like this are a great hub to access community members and have conversations about recovery services. Like most things in life, addiction is complex involving a multitude of factors that contribute to the addictive behavior. Drugs are the symptom, but the person is the real key to the solution within the equation. Lived experiences matter when looking at public health issues across the board. How people experience live greatly shapes how they decide to show up for it, especially in challenging times. If there are 343,000 Veterans who use illicit drugs, then effective and targeted programs need to be in place not only at VA systems but also in their surrounding communities. One of the great aspects of SSP programs is that it targets Veterans by how they are currently living with a substance use disorder, and while strengthening community engagement through public service.
Military culture and trauma are often associated with one another, but it isn’t always linked to deployment. That said, combat-related post-traumatic stress disorder (PTSD) is quite prevalent among active-duty service members, as well as Veterans. For service members nearing active-duty discharge, a diagnosis of PTSD may change the status of their discharge, greatly affecting the outcome of receiving services from the VA. The term “bad papers” is used within military culture to signify that a Veteran has been discharged unfavorably. A status discharge of other than-honorable is essentially the kiss of death because it means that a Veteran will not be able to access services through the VA. What is interesting about this status is that it is given for felonies, those absent without official leave (AWOL), desertion and Veterans with drug offenses. The issue then becomes the consequent behaviors of Veterans struggling with PTSD who turn to substance use to cope and who then also begin to have behavioral changes which affects level of performance on all fronts. Veterans carry an immense sense of pride for their service, and rightfully so. They have stepped in roles that most people don’t have the courage to do so. As an evolving clinician, seeing a Veteran struggle with PTSD due to the natural climate of what their duty demands of them, and then being shut out of benefits that are crucial to their mental health is just unacceptable. Discharge review boards really need to reconsider the criteria for evaluating Veterans who suffer from traumatic events. Not doing so sends a message that devalues the sacrifices that they have made which then perpetuates the stigma associated with their discharge status, but also reinforces the negative outlook of mental health within military culture. Veterans should not have to suffer in silence for enduring what was demanded of them and then be casted aside because their organization feels that their value has expired.
In 2016, over 1.5 million of the 5.5 million Veterans who entered the VA hospitals, had PTSD or other mental health diagnosis. That’s a staggering number especially when you consider the constant influx of Veterans who are returning home from deployment. Compared to the general population, suicide death rates are higher in Veterans, and furthermore female Veterans have a suicide rate of 35 per 100,000. Mental health services within the VA system have been on ongoing challenge as they try to meet the demand that Veterans need for crisis-intervention. As it is, mental health services are expensive for nonveterans, and even those who are insured may not have the adequate coverage to seek mental health services during a crisis episode. For Veterans returning home experiencing a mental health condition, this is disastrous as communities and VA systems both struggle to provide crisis stabilization and interventions. As a result, many Veterans experience depression on top of another mental health diagnosis like PTSD. Homelessness in Veterans is also increasing with more than 107,000 Veterans who are displaced. All of this is a perfect storm for a Veteran to feel like all hope is lost and consider suicide and reports reflect that with 21 Veterans, on average, dying of suicide every day. In society, there is a lot of talk about how all human beings are deserving of human equity. Human equity should include the ability to access mental health services (and healthcare as a whole), and the capability to navigate healthcare systems by having the support of organizations, communities, and effective public policy.
The military culture’s sphere of influence is completely different from civilian life. It is a complex system demanding everything military personnel can give, but it can often fall short when the time comes to giving back to Veterans. The sad truth is, Veterans often confront too many barriers when attempting to access appropriate timely care and treatment. It isn’t a secret that mental health disorders and other numerous challenges, such as substance use disorder, stem from military service-related experiences. Yet, systems in place for Veterans are inadequately structured to meet the numerous public health issues confronting Veterans and, subsequently, their families. Accessibility to healthcare services, including mental health, needs to encompass a wide net of effective policies and programs but also infused with the knowledge of how Veterans occupy the various systems that they live in and are affected by them. Too often in healthcare, clients are evaluated solely based off a diagnosis and without ever including who they are and their lived experiences. These are large, missed opportunities for clinicians to home in on invaluable information that can help formulate more effective treatment plans in conjunction with innovative and effective public policy. Hubs like VA systems are a great resource for Veterans, but we need to make sure the avenues of accessibility remain open for all Veterans that are eligible. It is very rare that a solution to a problem ever stands alone, and this perspective should continue to be a driver as community engagement and expansion in healthcare accessibility is needed. Veterans answered the call of duty without hesitation so now we must not drag our feet when Veterans need us the most in a war that poor public policy, lack of community programs and military culture has waged on them.
References:
Belperio, A,. Korshak,L., & Moy, E. (2020). Hepatitis C Treatment in Minority Veterans. Office of Health Equity. Retrieved online at https://www.va.gov/HEALTHEQUITY/Hepatitis_C_Treatment_in_Minority_Veterans.asp
Burek, Gregory, M.D. (2018). Military Culture: Working With Veterans. The American Journal of Psychiatry Residents’ Journal. Retrieved online at https://ajp.psychiatryonline.org/doi/pdf/10.1176/appi.ajp-rj.2018.130902
Centers for Disease Control & Prevention (2018). CDC Estimates Nearly 2.4 Million Americans Living with Hepatitis C. U.S. Department of Health & Human Services. Retrieved online at https://www.cdc.gov/nchhstp/newsroom/2018/hepatitis-c-prevalence-estimates-press-release.html
Duncan, M. S., Alcorn, C. W., Freiberg, M. S., So-Armah, K., Patterson, O. V., DuVall, S. L., ... & Brittain, E. L. (2021). Association between HIV and incident pulmonary hypertension in US Veterans: a retrospective cohort study. The Lancet Healthy Longevity.
HepMag (2019). Veterans and Hepatitis C. Retrieved online at https://www.hepmag.com/basics/hepatitis-c-basics/veterans
Hester, R. D. (2017). Lack of access to mental health services contributing to the high suicide rates among veterans. International journal of mental health systems, 11(1), 1-4.
Maguire, Elizabeth (2021). Providing clean syringes to Veterans who inject drugs. VAntage Point (Blog). Retrieved online at https://blogs.va.gov/VAntage/89943/providing-veterans-inject-drugs-clean-syringes/
Military Officers Association of America blog (2017). Veterans and Opioid Addiction. Retrieved online at https://www.moaa.org/content/publications-and-media/features-and-columns/health-features/veterans-and-opioid-addiction/#.YNxcrmTZy_0.twitter
Pebody.,R. (2018). Life expectancy for people living with HIV. AIDSmap. Retrieved online at https://www.aidsmap.com/about-hiv/life-expectancy-people-living-hiv
Schultz, Jennifer (2017, November 10). Veterans By the Numbers. The NCSL Blog. Retrieved online at https://www.ncsl.org/blog/2017/11/10/veterans-by-the-numbers.aspx#:~:text=There%20are%2018.8%20million%20veterans%20living%20in%20the,rise.%20Veterans%20tend%20to%20be%20older%20than%20nonveterans
Sico, J. J., Kundu, S., So‐Armah, K., Gupta, S. K., Chang, C. C. H., Butt, A. A., ... & Stewart, J. C. (2021). Depression as a Risk Factor for Incident Ischemic Stroke Among HIV‐Positive Veterans in the Veterans Aging Cohort Study. Journal of the American Heart Association, 10(13), e017637.
Sisk, R. (2021). ‘Dirty, Embarrassing Secret:’ Veterans with PTSD Struggle to Shed Stigma of Bad Paper Discharges. Military. Military.com. Retrieved online at https://www.military.com/daily-news/2021/04/21/dirty-embarrassing-secret-veterans-ptsd-struggle-shed-stigma-of-bad-paper-discharges.html
Substance Abuse and Mental Health Services Administration. (2020). 2019 National Survey on Drug Use and Health: Veteran Adults. Retrieved online at https://www.samhsa.gov/data/sites/default/files/reports/rpt31103/2019NSDUH-Veteran/Veterans%202019%20NSDUH.pdf
U.S Department of Veterans Affairs, (2020). Human Immunodeficiency Virus (HIV) - VA IS THE LARGEST SINGLE PROVIDER OF HIV CARE IN THE UNITED STATES. Fact sheet. Retrieved online at https://www.hiv.va.gov/pdf/HIV-program-factsheet.pdf
Wisely, Rene (2018). Why Are Hep C Infections Skyrocketing? Opioid Abuse to Blame. Michigan Medicine. Retrieved online at https://healthblog.uofmhealth.org/hep-c-infection-and-drug-abuse
Zuchowski, J. L., Hamilton, A. B., Pyne, J. M., Clark, J. A., Naik, A. D., Smith, D. L., & Kanwal, F. (2015). Qualitative analysis of patient-centered decision attributes associated with initiating hepatitis C treatment. BMC gastroenterology, 15(1), 1-10.
Biden Administration’s Healthcare Future is One of Promise & Peril
Last month, the Biden Administration issued a press release outlining a look toward the future of American health care policy. Priorities in the presser include ever elusive efforts around prescription drug pricing and items with steep price tags like expanding Medicare coverage to include dental, hearing, and vision benefits, a federal Medicaid look-alike program to fill the coverage gaps in non-expansion states, and extending Affordable Care Act (ACA) subsidies enhancements instituted under the American Rescue Plan (ARP) in March. Many of these efforts are tied to the upcoming $3.5 trillion reconciliation package.
President Biden renewed his call in support of the Democrats effort to negotiate Medicare prescription drug costs, enshrined in H.R. 3. Drug pricing reform has been an exceptional challenge despite relatively popular support among the voting public, in particular among seniors. The pharmseutical industry has long touted drug prices set by manufacturers do not represent the largest barriers to care and mandating lower drug costs would harm innovation and development of new products. Indeed, for most Americans, some form of insurance payer, public or private, is the arbiter of end-user costs by way of cost-sharing (co-pays and co-insurance payments). To even get to that point, consumers need to be able to afford monthly premiums which can range from no-cost to the enrollee to hundreds of dollars for those without access to Medicaid or federal subsidies. The argument from the drug-making industry giants is for Congress to focus efforts that more directly impact consumers’ own costs, not health care industry’s costs. Pharmaceutical manufacturers further argue mandated price negotiation proposals would harm the industry’s ability to invest the development of new products. To this end, the Congressional Budget Office (CBO) recently released a report giving some credence to this claim. The CBO’s report found immediate drug development would hardly be impacted as those medications currently “in the pipeline” would largely be safe, but a near 10% reduction in new drugs over the next 30 years. While new drug development has largely been focused on “personalized” medicine – or more specific treatments for things like cancer – implementing mRNA technology into vaccines is indeed a matter of innovation (having moved from theoretical to shots-in-arms less than a year ago). With a pandemic still bearing down on the globe, linking the need between development and combating future public health threats should be anticipated.
The administration’s effort to leverage Medicare isn’t limited to drug pricing. Another tectonic plate-sized move would seek to expand “basic” Medicare to include dental, hearing, and vision coverage. Congressional Democrats, while generally open to the idea, are already struggling with timing of such an expansion, angering Senator Bernie Sanders (I-VT) by suggesting a delay until 2028. While any patient with any ailments related to their oral health, hearing, and vision will readily tell you these are critical and necessary coverages, even some of the most common of needs, the private health care insurance industry generally requires adult consumers to get these benefits as add-ons and the annual benefit cap is dangerously low (with dental coverage rarely offering more than $500 in benefit and vision coverage capping at one set of frames, both with networks so narrow as to be near meaningless for patients with transportation challenges). While the ACA expanded a mandatory coverage for children to include dental and vision benefits in-line with private adult coverage caps, the legislation did nothing to mandate similar coverages for adults and did not require private payers to make access to these types of care more meaningful (expanded networks and larger program benefits to more accurately match costs of respective care).
The other two massive proposals the Biden Administration is seeking support for, more directly impact American health care consumers than any other effort from the administration: maintaining expanded marketplace subsidies and a federal look-a-like for people living in the 12 states that have not yet expanded Medicaid under the ACA’s Medicaid expansion provisions. The administration has decent data to back this idea, as the Centers for Disease Control and Prevention released a report showing a drop in the uninsured rate from 2019 to 2020 by 1.9 million people, largely attributed by pandemic-oriented programs requiring states to maintain their Medicaid rolls. The administration and Congressional Democrats are expected to argue subsequently passed legislation allowing for expanded subsidies and maintained Medicaid rolls improved access to and affordability of care for vulnerable Americans during the pandemic. As the nation rides through another surge of illness, hospitalizations, and death from the same pandemic “now isn’t the time to stop”, or some argument along those lines, will likely be the rhetoric driving these initiatives.
Speaking of the pandemic, President Biden outlined his administration’s next steps in combating COVID-19 on Thursday, September 9th. The six-pronged approach, entitled “Path out of the Pandemic”, includes leveraging funding to support mitigation measures in schools (including back-filling salaries for those affected by anti-mask mandates and improving urging the Food and Drug Administration [FDA] to authorize vaccines for children under the age of 12), directing the Occupational Safety and Health Administration (OSHA) to issue a rule mandating vaccines or routinized testing for employers with more than 100 employees (affecting about 80 million employees) and mandating federally funded health care provider entities to require vaccination of all staff, pushing for booster shots despite the World Health Organization’s call for a moratorium until greater global equity in access can be attained, supporting small businesses through previously used loan schemes, and an effort to expand qualified health care personnel to distribute COVID-19 related care amid a surge threatening the nation’s hospitals ability to provide even basic care. Notably missing from this proposal are infrastructure supports for schools to improve ventilation, individual financial support (extension of pandemic unemployment programs or another round of direct stimulus payments), longer-term disability systems to support “long-COVID” patients and any yet-unknown post-viral syndromes, and housing support – which is desperately needed as the administration’s eviction moratorium has fallen victim to ideological legal fights, states having been slow to distribute rental assistance funds, and landlords are reportedly refusing rental assistance dollars in favor of eviction. While the plan outlines specific “economic recovery”, a great deal is left to be desired to ensure families and individuals succeed in the ongoing pandemic. Focusing on business success has thus far proven a limited benefit to families and more needs to be done to directly benefit patients and families navigating an uncertain future.
President Biden did not address global vaccine equity in his speech, later saying a plan would come “later”. The problem, of course, is in a viral pandemic, variant development has furthered risks to wealthy countries with robust vaccine access and threatened the economic future of the globe.
To top off all of this policy-making news, Judge Reed O’Connor is taking another swing at dismantling some of the most popular provisions of the ACA. Well, rather, yet another plaintiff has come to the sympathetic judge’s court in an effort to gut the legislation’s preventative care provisions by both “morality” and “process” arguments in Kelley v. Becerra. The suit takes exception to a requirement that insurers must cover particular preventative care as prescribed by three entities within the government (the Health Resources Services Administration – HRSA, the Advisory Committee on Immunization Practices – ACIP, and the Preventative Services Takes Force – PSTF), which require coverage of contraceptives and pre-exposure prophylaxis (PrEP) with no-cost sharing to the patient, among a myriad of other things – including certain vaccine coverage. By now, between O’Connor’s rabid disregard for the rights of lesbian, gay, bisexual, and transgender Americans and obsessive effort to dismantle the ACA at every chance he can – both to his own humiliation after the Supreme Court finally go their hands on his rulings – Reed O’Connor may finally have his moment to claim a victory – I mean – the plaintiffs in Kelley may well succeed due to the Supreme Court’s most recent makeover.
As elected officials are gearing up for their midterm campaigns, how these next few months play out will be pretty critical in setting the frame for public policy “successes” and “failures”. Journalists would do well to tap into the expertise of patient advocates in contextualizing the real-world application of these policies, both during and after budget-making lights the path to our future – for better or worse.
A Different Booster: HBV Vaccines among PLWHA
Because of the shared transmission vectors between HIV and Hepatitis B (HBV), the rate of co-infection is about 10% in the United States, according to the Centers for Disease Control and Prevention (CDC). As a result, people living with HIV (PLWHA) are more likely to experience adverse health impacts including cirrhosis and certain types of liver cancers. A small study conducted in Chile took a look at the recommended HBV vaccine schedule among adults living with HIV and HBV antibody uptake and potentially finding cause for a “high dose” fourth shot to be added into the series for PLWHA.
A giant asterisk belongs on the study’s findings, labeled “deserves further study consideration”. Despite being double-blinded, the study’s greatest weakness included participant pool size (right around 100 participants) and clinical selection criteria (which remains an issue in clinical trial work, generally speaking). In order to be considered for the study, participants generally had to present with an undetectable HIV viral load and no other comorbidities, ruling out application of the resulting data to most PLWHA and especially most long-term survivors or people experiencing barriers to care or medication adherence concerns – or those most likely to be impacted by HIV and HBV co-infection.
The study sought to examine the need for revaccination among PLWHA. Of note, the CDC’s “Pink Book” on HBV does not recommend “boosters” unless a particular “low” threshold of HBV antibodies is met, nor does the publication recommend for routinized serological testing among people who have previously received a vaccine. Therein lies a program and policy problem. We’ll get to that in a moment.
As a result of selection bias favoring those with more ideal circumstances, few participants dropped out of the trial. The study itself found that a fourth and “stronger” dose of vaccine improved antibody responses among people with “well controlled” HIV with an improved HBV antibody response from 50.9% in the low-dose arm of the study to 72% among the high-dose arm of the trial. After a one-year follow up, 80% of participants of the high-dose arm still had sufficient antibody titers, whereas only 39% of the standard-dose arm still had sufficient antibodies for protection.
While Ryan White and CDC funded clinical care programs for PLWHA require HBV monitoring and vaccination efforts as part of their grant funding, few entities necessarily do and almost no private providers do. Federally-funded providers may screen upon intake or initial labs but maintenance screening is not a priority in terms of clinical data collected on a given patient. Even on-site audits from these funders can sometimes look like reviewing particular case files and discussing details but the HBV conversation is not pressing. Rather, a review of intake data can suffice depending on the clinical auditor/consultant (site-visits and audits are often conducted under the supervision of the funding agency but only actually audited by consultants, including staff from other funded clinics).
Public funders aiming to end HBV and the unjust circumstances in which PLWHA are not educated by their providers on the other risks to their health should shift some focus to emphasize the need for preventative care – especially vaccines. Provider education for these publicly funded clinics should include the need to routinize HBV antibody monitoring not just as a concern on behavioral risk factors continuing in a client’s life but because HBV immunity is clearly not necessarily a given, regardless of prior vaccination history.
While the study suggests the need for investigating further, with regard to efficacy of HBV vaccines among PLWHA, the larger question - given the nation-wide rush for another vaccine (and boosters) - creating more robust standards of care among a population known to have immunological “memory-loss” due to the particular cells “attacked” by HIV seems to be in order. Part and parcel to that is tying this level of necessary education to funding and licensure could improve the quality of care PLWHA receive, especially those of low-income and otherwise marginalized identities.
Amid HIV Outbreaks, Covid-19 Fractures Existing Public Health Efforts
“Every health disparity map in the United States is the same,” Alex Vance, Senior Director of Advocacy and Public Policy at the International Association of Providers in AIDS Care, has repeatedly stated when discussing the COVID-19 pandemic and the very real risks of “back sliding” in our moderate advancements in the United States’ effort to End the HIV Epidemic. There’s no better way to demonstrate this than by showing you.
As another “wave” of Covid-19 ravages the country, increasing cases, hospitalization rates, and eventually deaths, existing public health needs around HIV, viral hepatitis, and sexually transmitted infections continue to be strained, pushed to the back burner and most often in geographic areas where we need to be able to juggle more, not less; particularly across the South. Unlike Covid-19 outbreaks, which receive fairly immediate attention in terms of reporting and response, HIV outbreaks can and do often take a year or more to notice and begin action to address. While there’s concern about juggling the demands of addressing concurrent pandemics, some (certainly not all) of this reporting and response is beginning to speed up with regard to HIV.
West Virginia’s Kanawha County HIV outbreak is considered a 2020 outbreak, though just recently received a report from the Center’s for Disease Control & Prevention (CDC) on next steps and recommendations to address. Despite these recommendations including supporting syringe exchange programs and community-based services, local officials continue a politically oriented response by seeking to limit the support of syringe exchange programs, threatening their ability to operate and aid in addressing the needs of the local community. Another outbreak in Michigan’s Upper Peninsula was identified in July of this year, with rural Kalkaska County reporting a higher rate of new HIV diagnoses than even Detroit. Kalkaska County is so similarly situated to Scott County, Indiana, it’s striking. Louisville, Kentucky has reported almost as many new HIV diagnoses in the first half of 2021 as the area does annually. And Duluth, outside of Saint Louis, Minnesota, joined neighboring Hennepin and Ramsey Counties with declared outbreaks tied to 2019 and 2018, respectively.
What’s important to note about 2020’s marked reduction in HIV screenings is not just the delay in newly diagnosing people living with HIV, but the lack of ability to link people to care upon diagnosis, beginning antiretroviral therapy and taking steps to reduce the person’s viral load. With at-home testing being the substitute offering, linkage to care and counseling for people self-testing may be hampered according to some concerned advocates. Achieving viral suppression also reduces the possibility of transmitting HIV by way of sexual contact to zero (“Undetectable = Untransmittable”) – creating a process where people living with HIV are not just a patient group needing identification, but play a critical role in preventing new transmissions.
In reviewing the possibilities of delayed care and delayed screening, public health officials and advocates should remember a new diagnosis is not necessarily indicative of a new transmission. A potential problem in and of itself, in assessing Covid-19 disruptions in screening and care, is the possibility of a “bottle neck” of new testing revealing new HIV diagnoses which otherwise might have been identified in the previous year if not for stay-at-home orders, education and public awareness campaigns, and community health care providers having had to take a step or shift gears entirely from HIV to Covid-19. It will take years for us to truly understand the breadth and reach of Covid-19 on the world’s only concurrent pandemic, even in the “most advanced country in the world”.
What can already be well-appreciated and should be well-understood is we cannot afford to keep asking community-based health care providers and community partners in combatting the domestic HIV epidemic to keep sacrificing HIV screening and linkage to care in order to address Covid-19. What must be prioritized is funding, programming, training, and most importantly hiring of new talent in addition to existing programs in order to address both sets of needs.
As I summarized in a previous blog, capacity has been breached, we cannot afford to keep asking public health to do more with less and expect to succeed in addressing Covid-19, HIV, viral hepatitis, STIs, or the opioid epidemic.