Watch 02: April 2024
The HIV/HCV Co-Infection Watch is a project of the Community Access National Network (CANN) designed to research, monitor, and report on HIV and Hepatitis C (HCV) co-infection in the United States. The April 2024 Watch includes timely updates herein. To read the project disclaimer and/or methodology, CLICK HERE.
1. FINDINGS
The following is a summary of the key findings for April 2024:
AIDS Drug Assistance Programs:
There are 56 state and territorial AIDS Drug Assistance Programs (ADAPs) in the United States, 47 of which offer some form of coverage for Hepatitis C (HCV) treatment. Of those programs, 45 have expanded their HCV coverage to include the Direct-Acting Antiviral (DAA) regimens that serve as the current Standard of Care (SOC) for Hepatitis C treatment. Two (2) programs offer only Basic Coverage and nine (9) programs offer No Coverage. One (1) program covers only a single Direct-Acting Antiviral. Three (3) territories – American Samoa, Marshall Islands, and Northern Mariana Islands – are not accounted for in this data. A state-by-state Drug Formulary breakdown of coverage is included in the April 2024 Updates, with accompanying drug-specific maps in Figures 1 – 10.
Medicaid Programs:
There are 59 state and territorial Medicaid programs in the United States, and data is represented for all 50 states and the District of Columbia. As of October 01, 2016, all 50 states and the District of Columbia offer Expanded Coverage. A state-by-state PDL breakdown of coverage is included in the January 2024 Updates, with accompanying drug-specific maps in Figures 11 – 20.
Harm Reduction Programs:
Every state and territory in the United States currently provides funding for low-income people living with substance abuse issues to enter state-funded rehabilitation services (National Center for Biotechnology Information, n.d.). Forty-four (44) states, the District of Columbia and two (2) territories currently have Syringe Services Programs (SSPs) in place, regardless of the legality. Fifty (50) states and the District of Columbia have health department distribution programs for Naloxone and/or allow Medicaid coverage of Naloxone. While Federal prohibits the possession and consumption of certain controlled substances, two (2) states explicitly authorize or have authorized pilot projects for Safe Consumption Sites (SCSs); SCSs exist to provide injection drug users (or those whose prescriptions require injection) with clean syringes and/or in exchange for used ones, offer wound care supplies, allow for injection drugs users to consume drugs, offer infectious disease screening, and other linkage to care opportunities. State Paraphernalia Laws (SPLs) have long prohibited possession of certain drugs use related materials, including harm reduction materials like fentanyl testing strips; forty-nine (49) states have modernized their criminal codes to allow for possession of testing strips and may also have health department programs distributing testing strips. Twenty-five (25) states and the District of Columbia have Good Samaritan laws or statutes that provide some level of protection for those seeking or giving assistance during a drug overdose, regardless of possession of controlled substance or consumption of illegal or illicit substances. Forty-nine (49) states and the District of Columbia require, through legislative action or regulatory or licensing bodies, that prescribing physicians to attend mandatory and continuing opioid prescribing or harm reduction education sessions (otherwise known as Prescriber Education laws). A state-by-state program breakdown is included in the January 2024 Updates, with accompanying drug-specific maps in Figures 21-26.
2. AIDS DRUG ASSISTANCE PROGRAMS (ADAPs) & HCV THERAPIES
Of the 56 respective state and territorial ADAPs, only 8 (KS, KY, OH, UT, VT, GU, PW, VI) do not offer any coverage for HCV drug therapies. States whose formularies are not available on the state-run website have been checked against the most recent National Alliance of State and Territorial AIDS Directors (NASTAD) formulary database (last updated January 1, 2024). The data presented are current as of April 24, 2024.
April 2024 Updates:
Basic Coverage
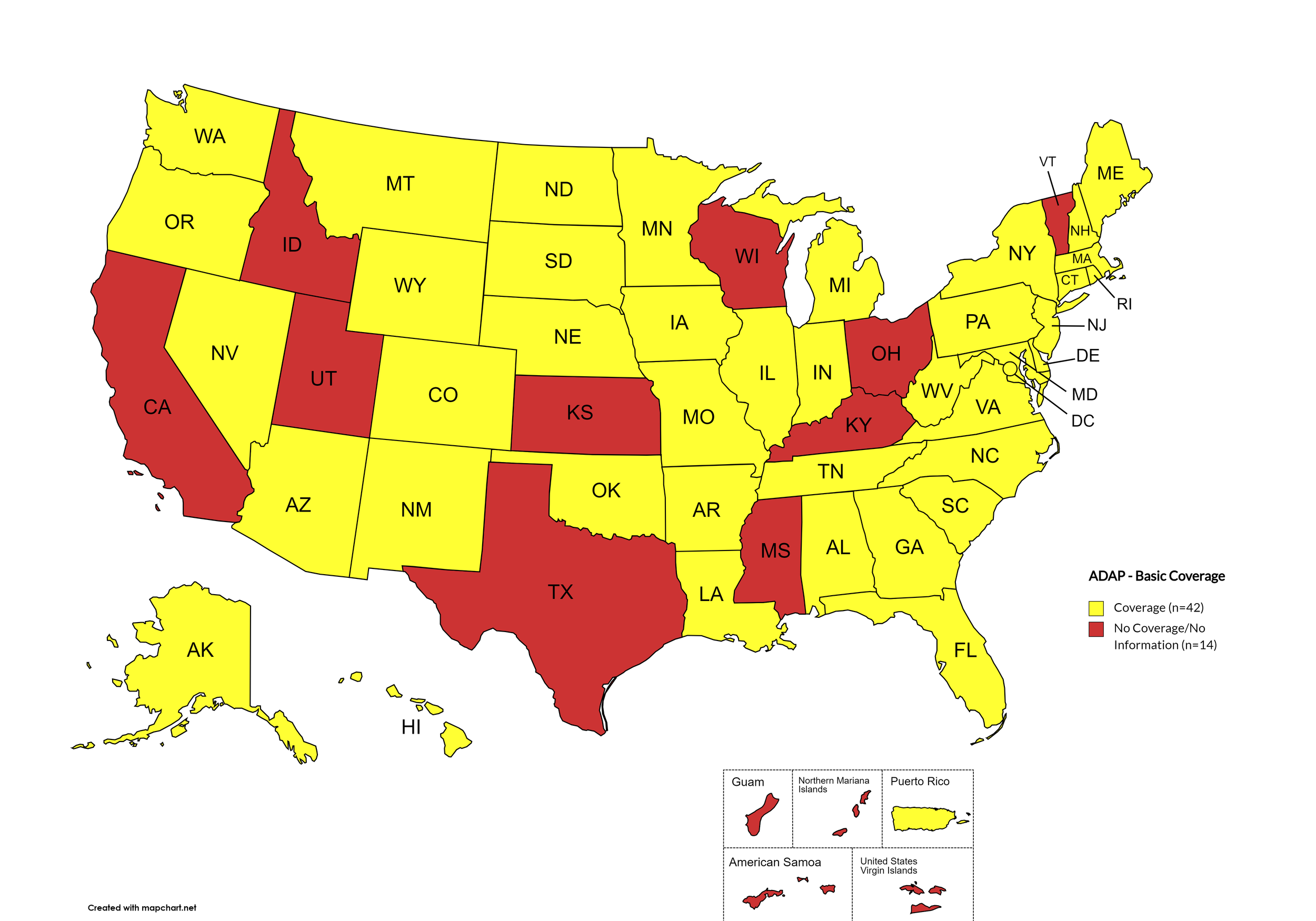
States with Basic HCV Medications Coverage: AL, AK, AZ, AR, CO, CT, DE, FL, GA, HI, IL, IN, IA, LA, ME, MD, MA, MI, MN, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OK, OR, PA, RI, SC, SD, TN, VA, WA, WV, WY, D.C.
States without Basic HCV Medications Coverage: CA, ID, KS, KY, MS, OH, TX, UT, VT, WI
Territories with Basic HCV Medications Coverage: P.R.
Figure 1. April 2024 ADAP Coverage - Basic
Map Key: Yellow = Basic Coverage; Red = No Basic Coverage/No Information regarding Basic Coverage
Sovaldi
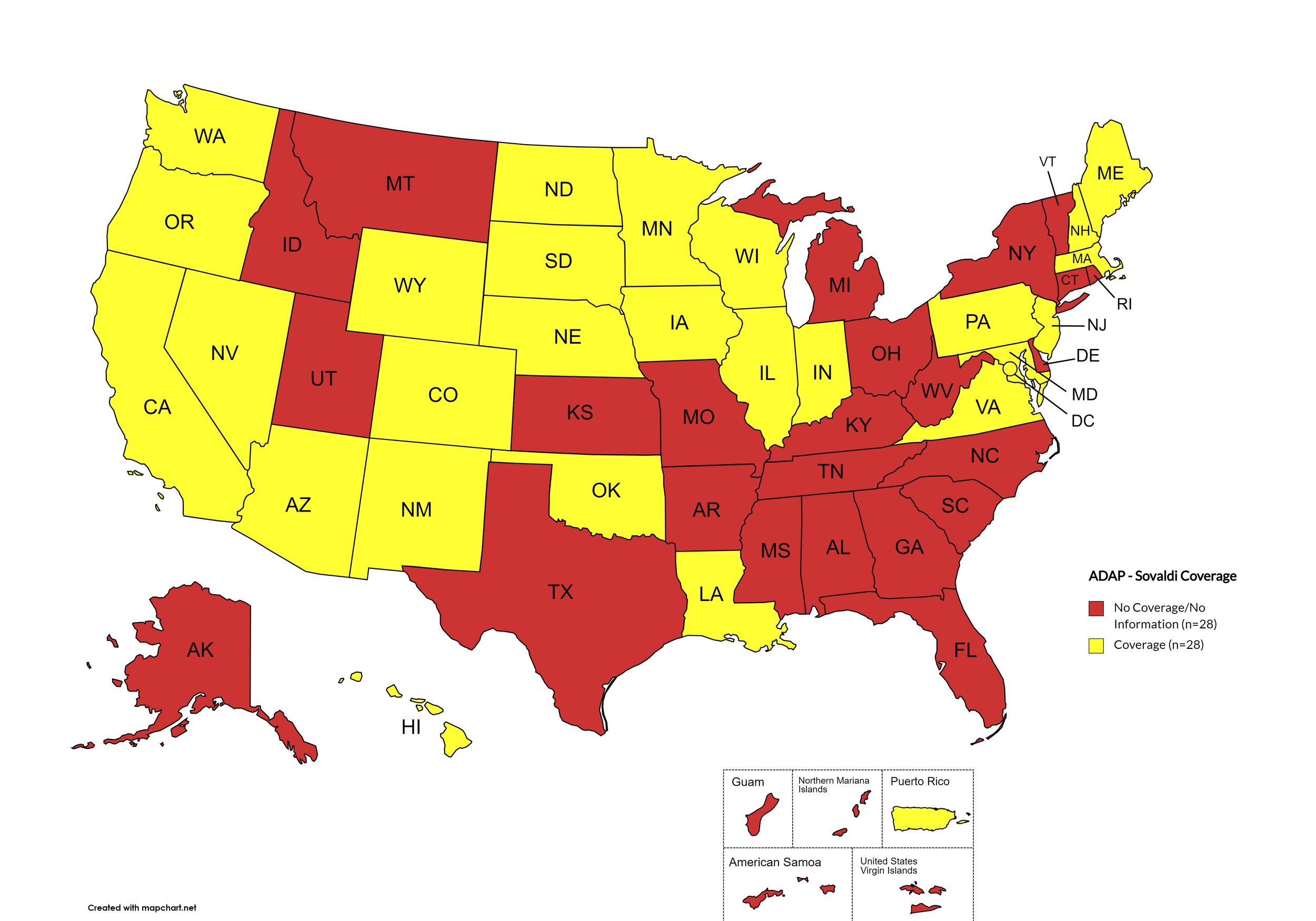
States with Sovaldi Coverage: AZ, CA, CO, HI, IL, IN, IA, LA, ME, MD, MA, MN, NE, NV, NH, NJ, NM, ND, OK, OR, PA, SD, VA, WA, WI, WY, D.C.
States without Sovaldi Coverage: AL, AK, AR, CT, DE, FL, GA, ID, KS, KY, MI, MS, MO, MT, NY, NC, OH, RI, SC, TN, TX, UT, VT, WV
Territories with Sovaldi Coverage: P.R.
Figure 2. April 2024 ADAP Coverage - Sovaldi
Map Key: Yellow = Sovaldi Coverage; Red = No Sovaldi Coverage/No Information regarding Sovaldi Coverage
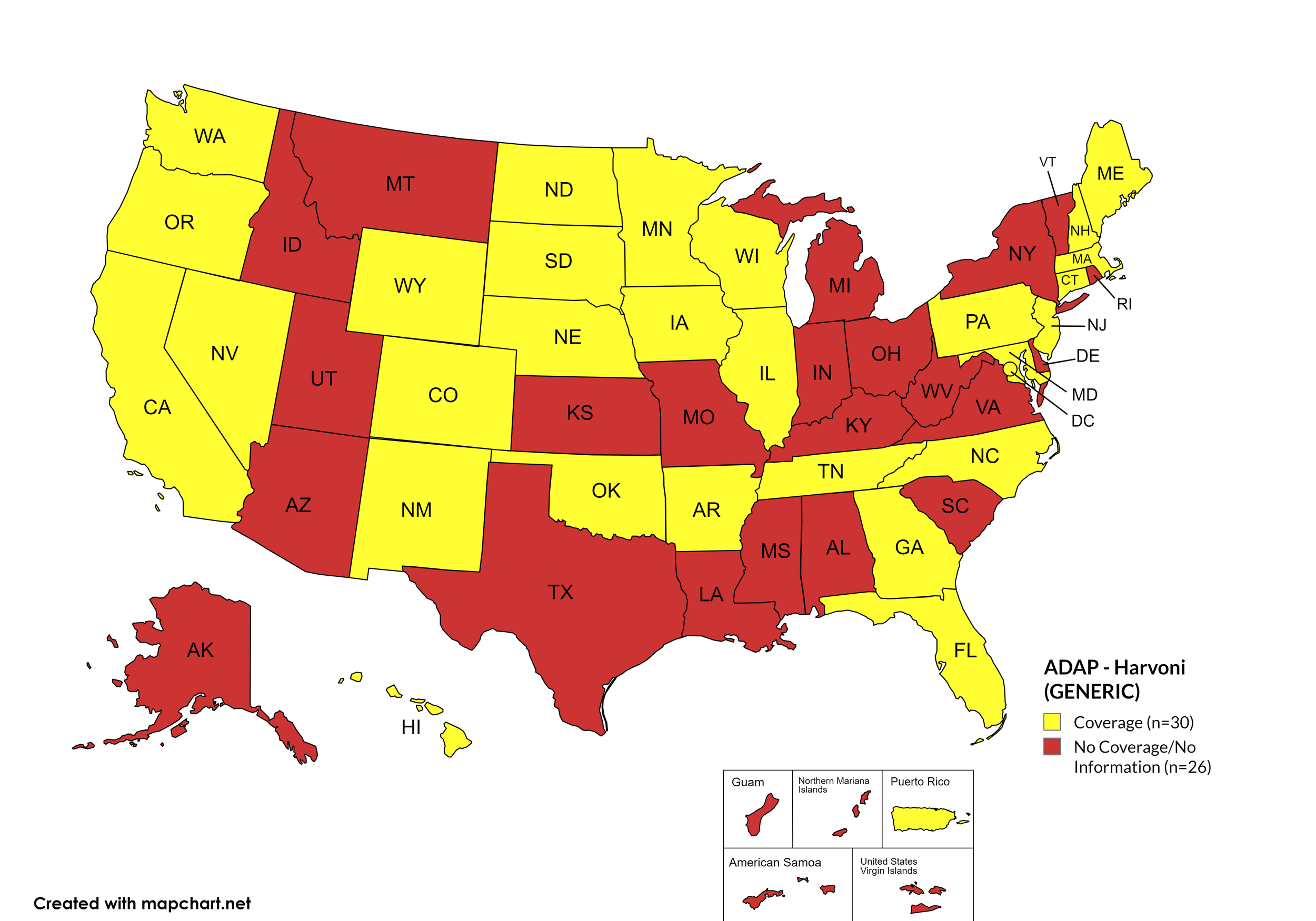
Harvoni
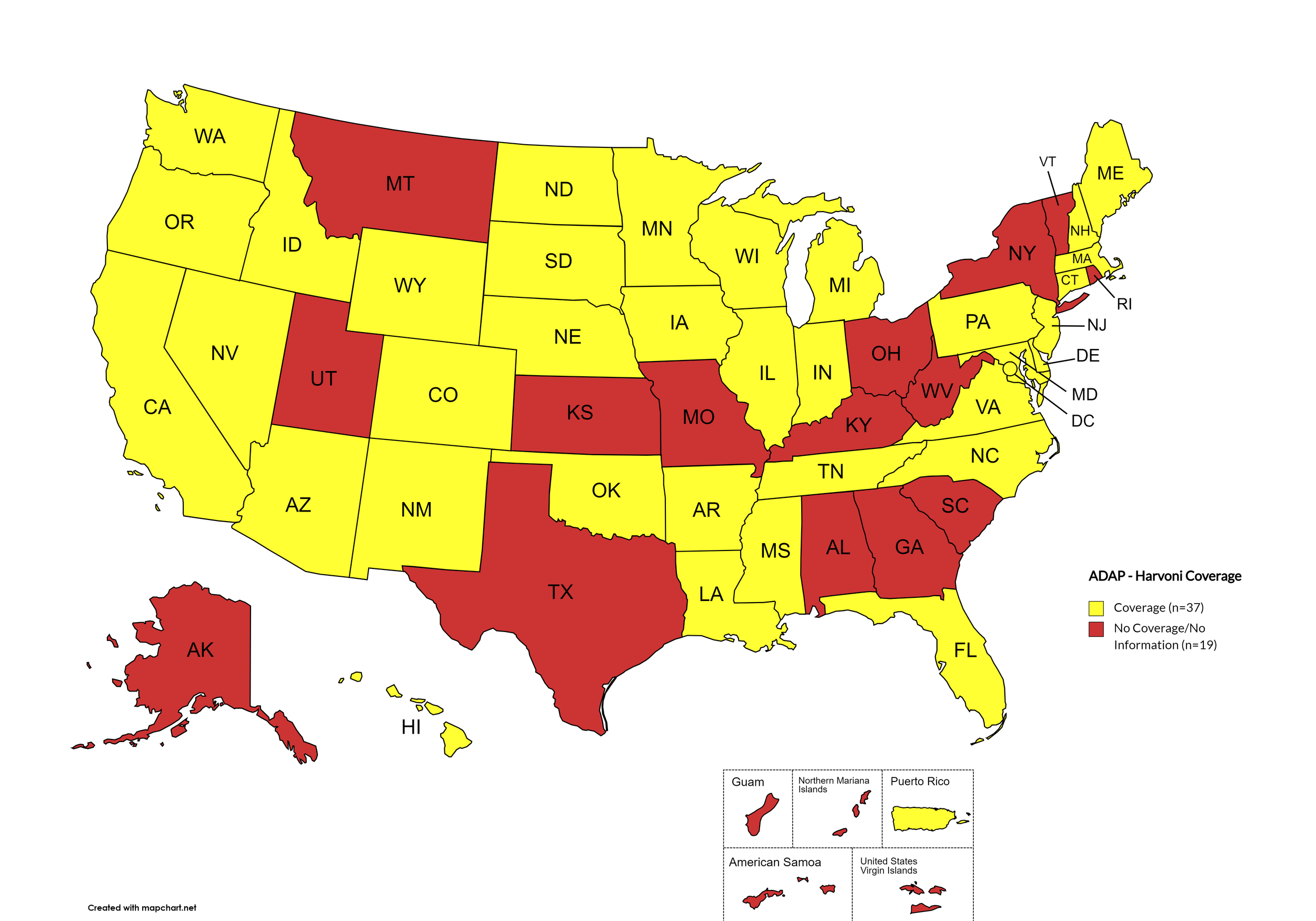
States with Harvoni Coverage: AZ, AR, CA, CO, CT, DE, FL, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, NE, NV, NH, NJ, NM, NC, ND, OK, OR, PA, SD, TN, VA, WA, WI, WY, D.C.
States without Harvoni Coverage: AL, AK, GA, KS, KY, MO, MT, NY, OH, RI, SC, TX, UT, VT, WV
Territories with Harvoni Coverage: P.R.
Figure 3. April 2024 ADAP Coverage - Harvoni
Map Key: Yellow = Harvoni Coverage; Red = No Harvoni Coverage/No Information regarding Harvoni Coverage
Zepatier
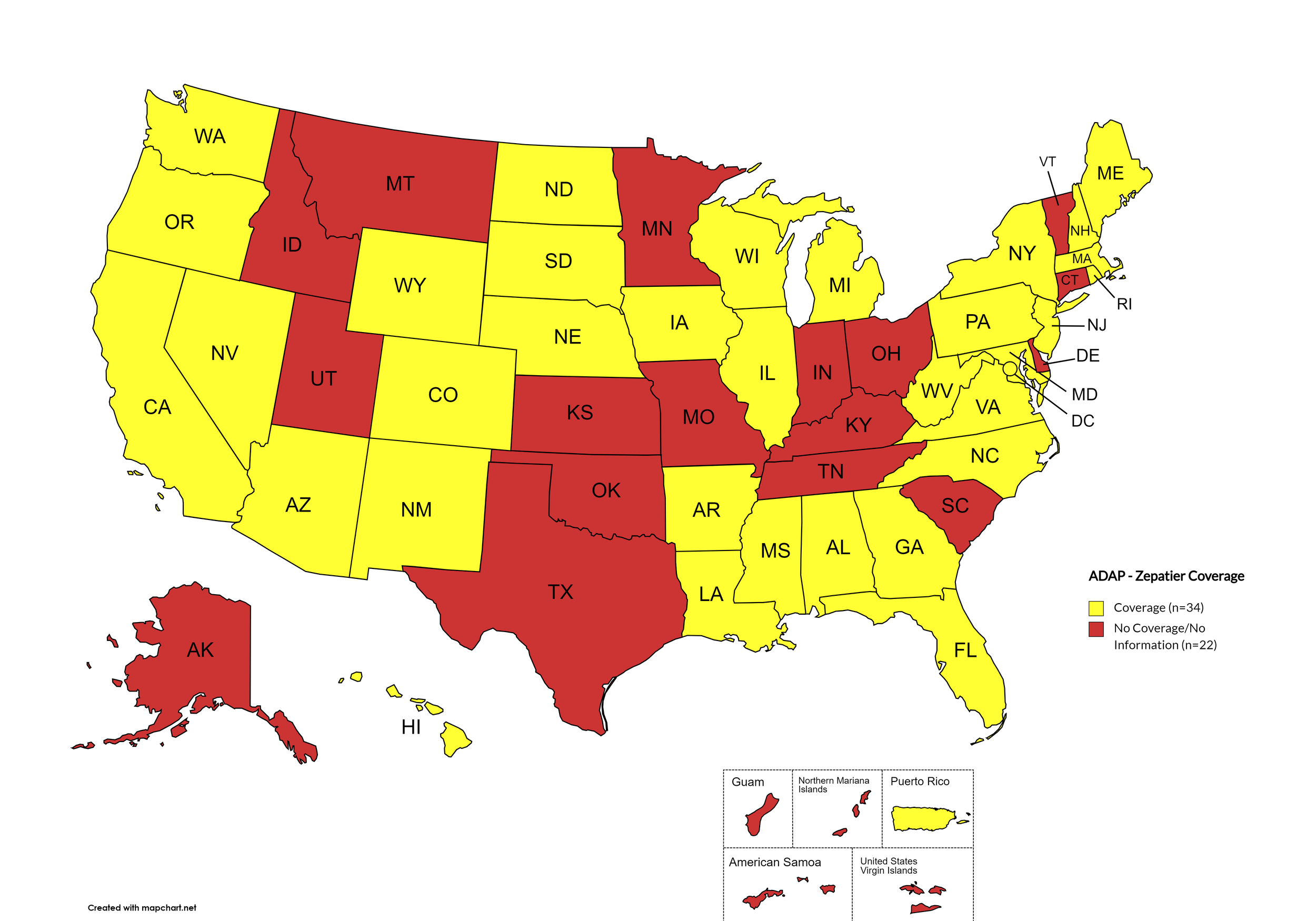
States with Zepatier Coverage: AL, AZ, AR, CA, CO, FL, GA, HI, IL, IA, LA, ME, MD, MA, MI, MS, NE, NV, NH, NJ, NM, NY, NC, ND, OR, PA, SD, VA, WA, WV, WI, WY, D.C.
States without Zepatier Coverage: AK, CT, DE, ID, IN, KS, KY, MN, MO, MT, OH, OK, RI, SC, TN, TX, UT, VT
Territories with Zepatier Coverage: P.R.
Figure 4. April 2024 ADAP Coverage - Zepatier
Map Key: Yellow = Zepatier Coverage; Red = No Zepatier Coverage/No Information regarding Zepatier Coverage
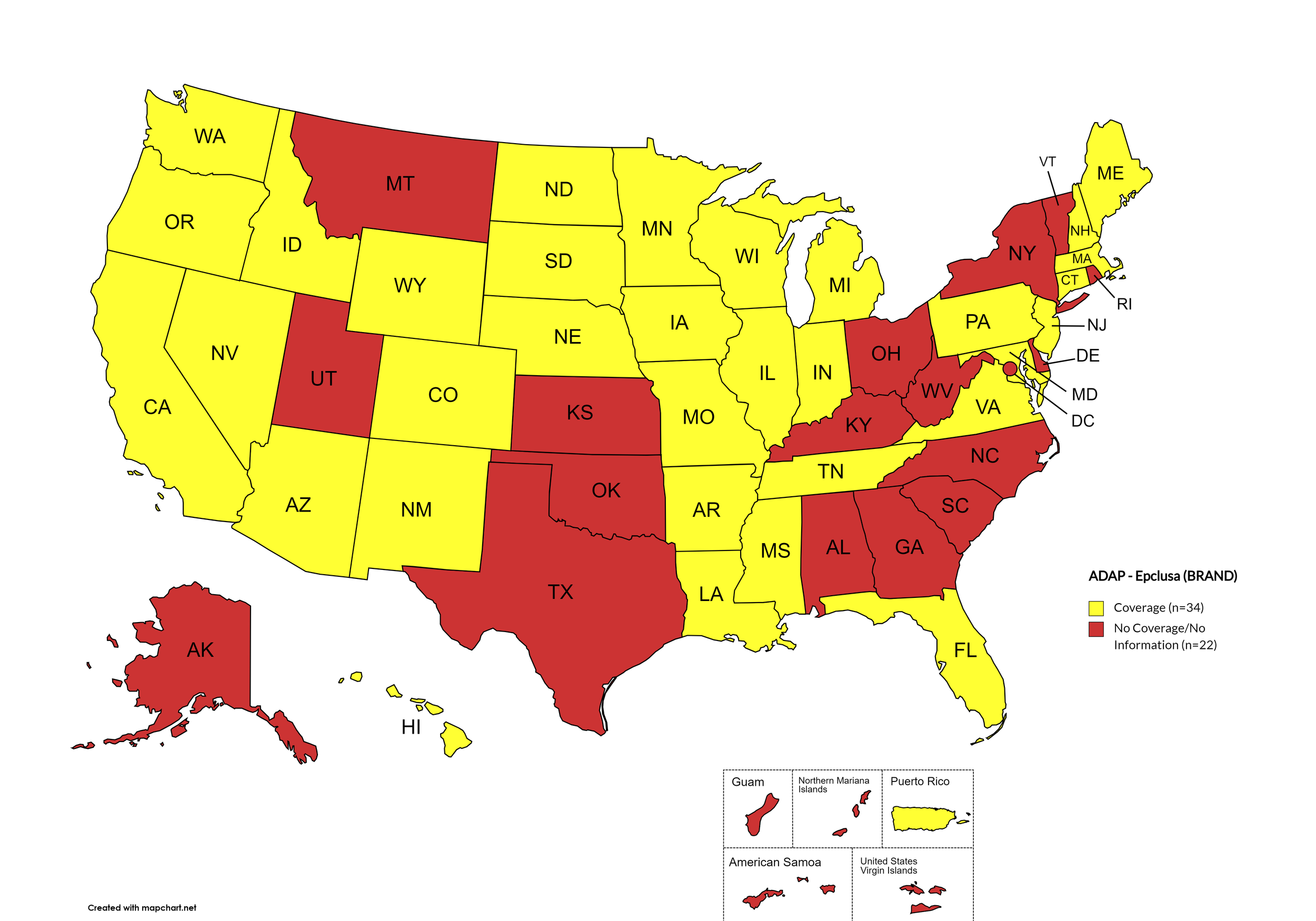
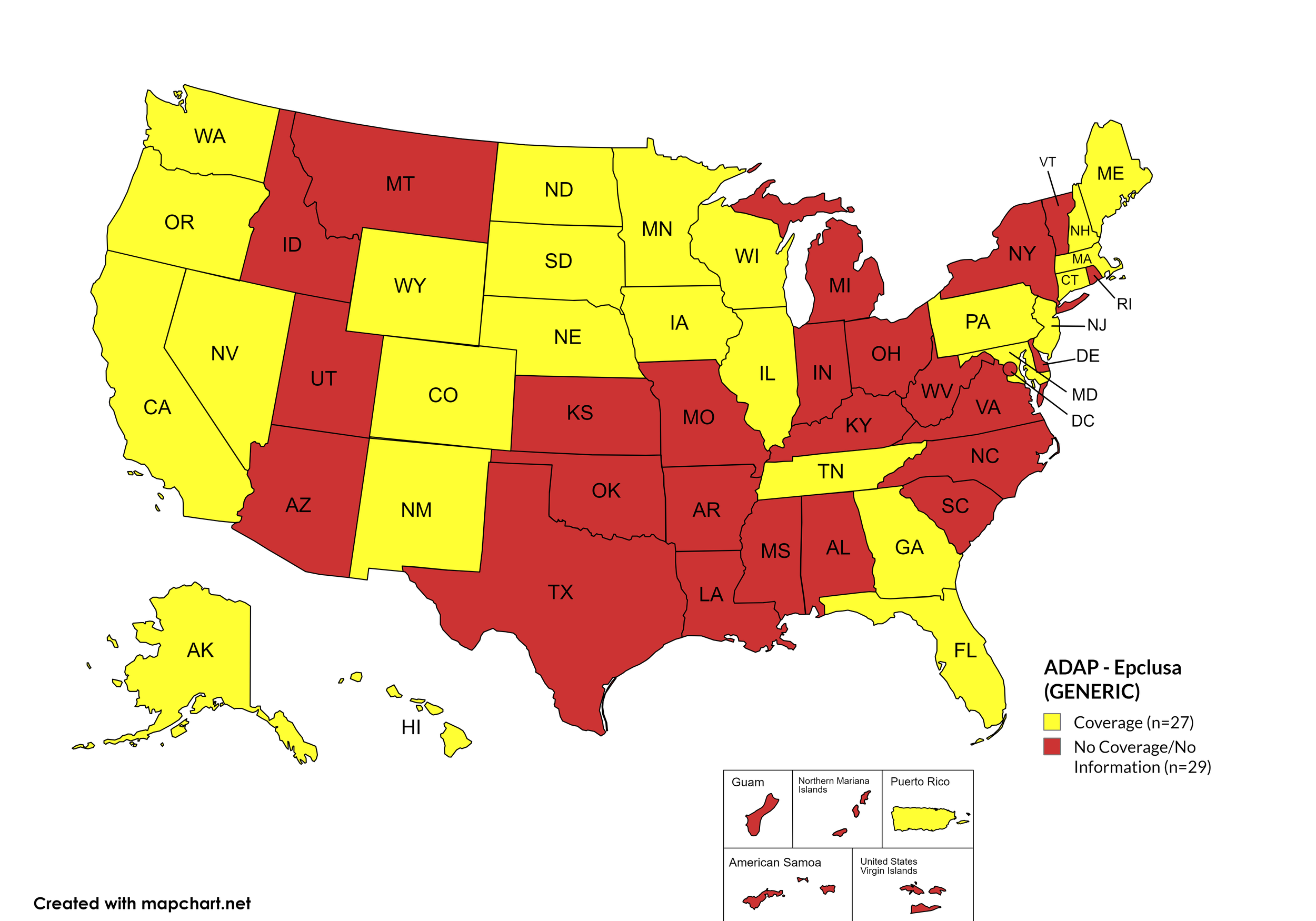
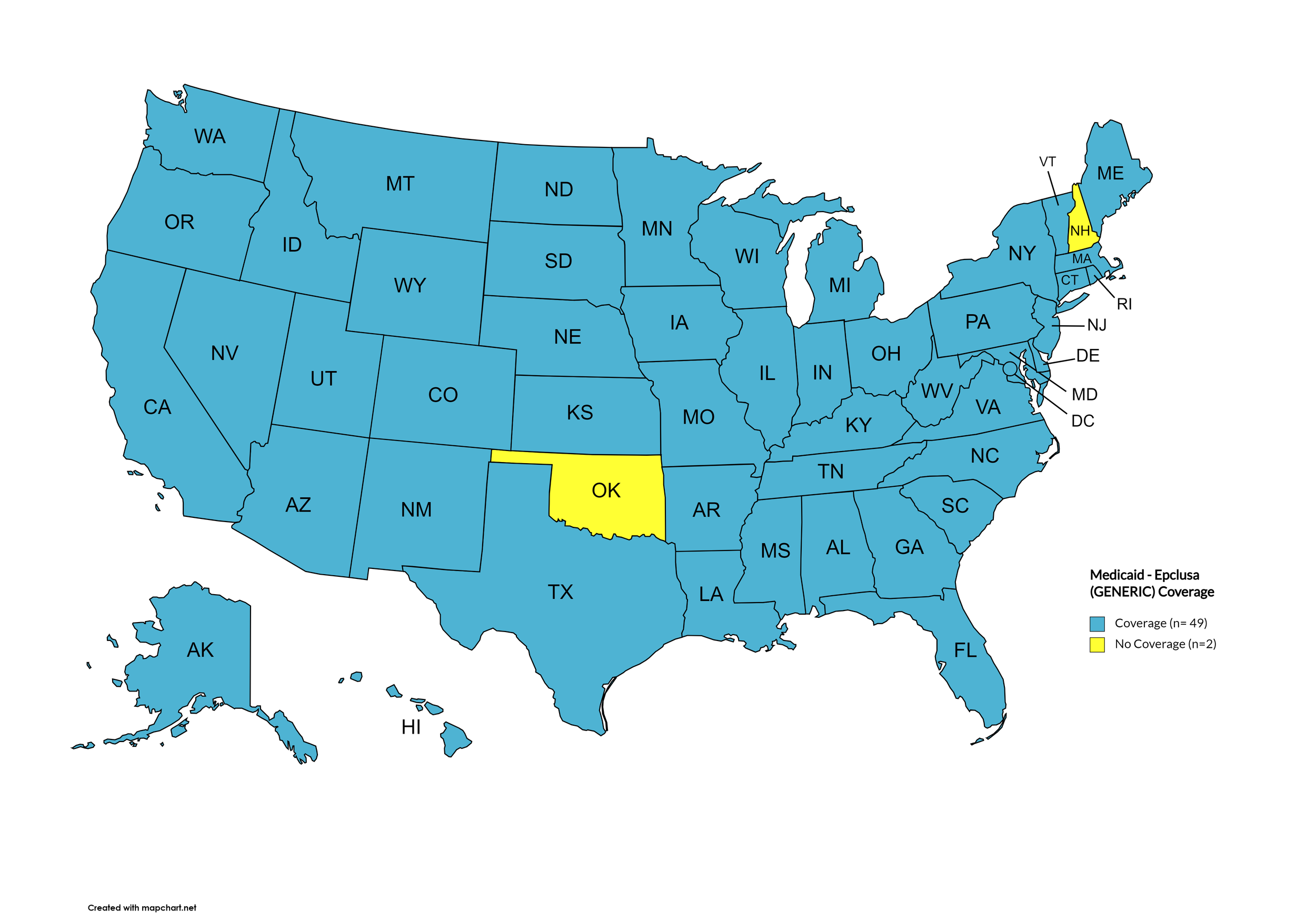
Epclusa
States with Epclusa Coverage: AZ, AR, CA, CO, CT, FL, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, MO, NE, NV, NH, NJ, NM, ND, OR, PA, SD, TN, VA, WA, WI, WY
States without Epclusa Coverage: AL, AK, DE, GA, KS, KY, MT, NY, NC, OH, OK, RI, SC, TX, UT, VT, WV, D.C.
Territories with Epclusa Coverage: P.R.
Figure 5. April 2024 ADAP Coverage - Epclusa
Map Key: Yellow = Epclusa Coverage; Red = No Epclusa Coverage/No Information regarding Epclusa Coverage
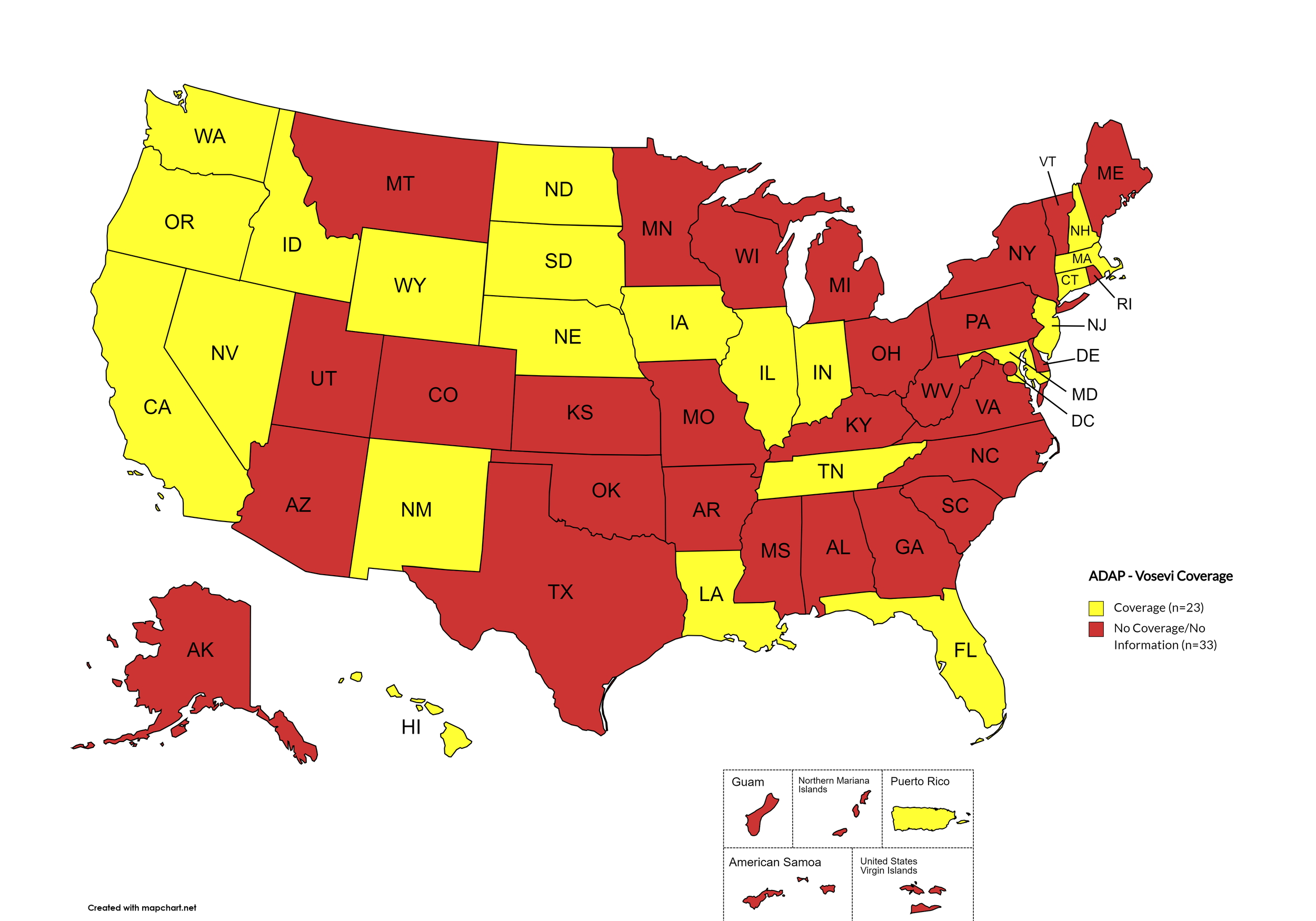
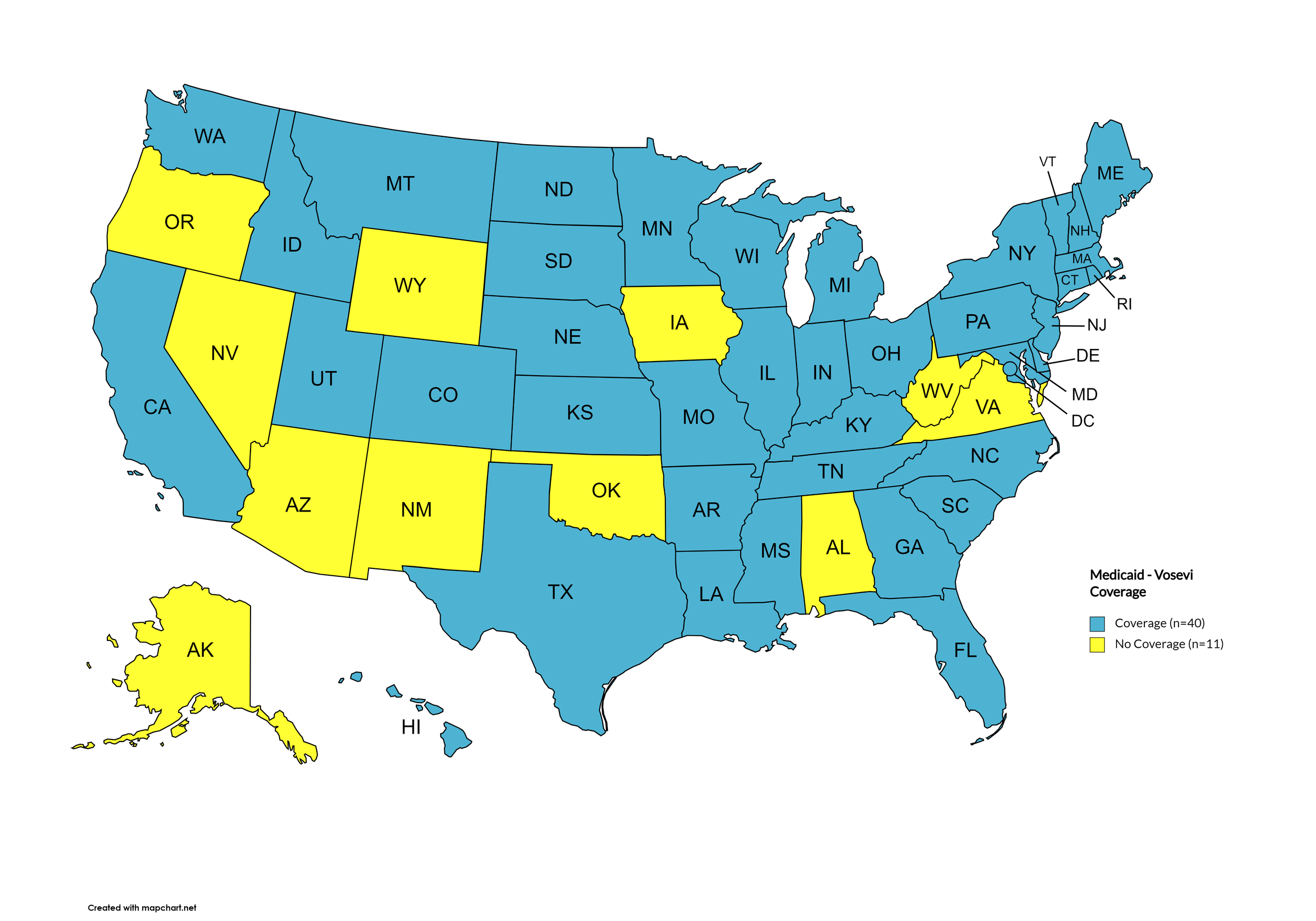
Vosevi
States with Vosevi Coverage: CA, CT, FL, HI, ID, IL, IN, IA, LA, MD, MA, NE, NV, NH, NJ, NM, ND, OR, SD, TN, WA, WY
States without Vosevi Coverage: AL, AK, AZ, AR, CO, DE, GA, KS, KY, ME, MI, MN, MS, MO, MT, NY, NC, OH, OK, PA, RI, SC, TX, UT, VT, VA, WV, WI, D.C.
Territories with Vosevi Coverage: P.R.
Figure 6. April 2024 ADAP Coverage - Vosevi
Map Key: Yellow = Vosevi Coverage; Red = No Vosevi Coverage/No Information regarding Vosevi Coverage
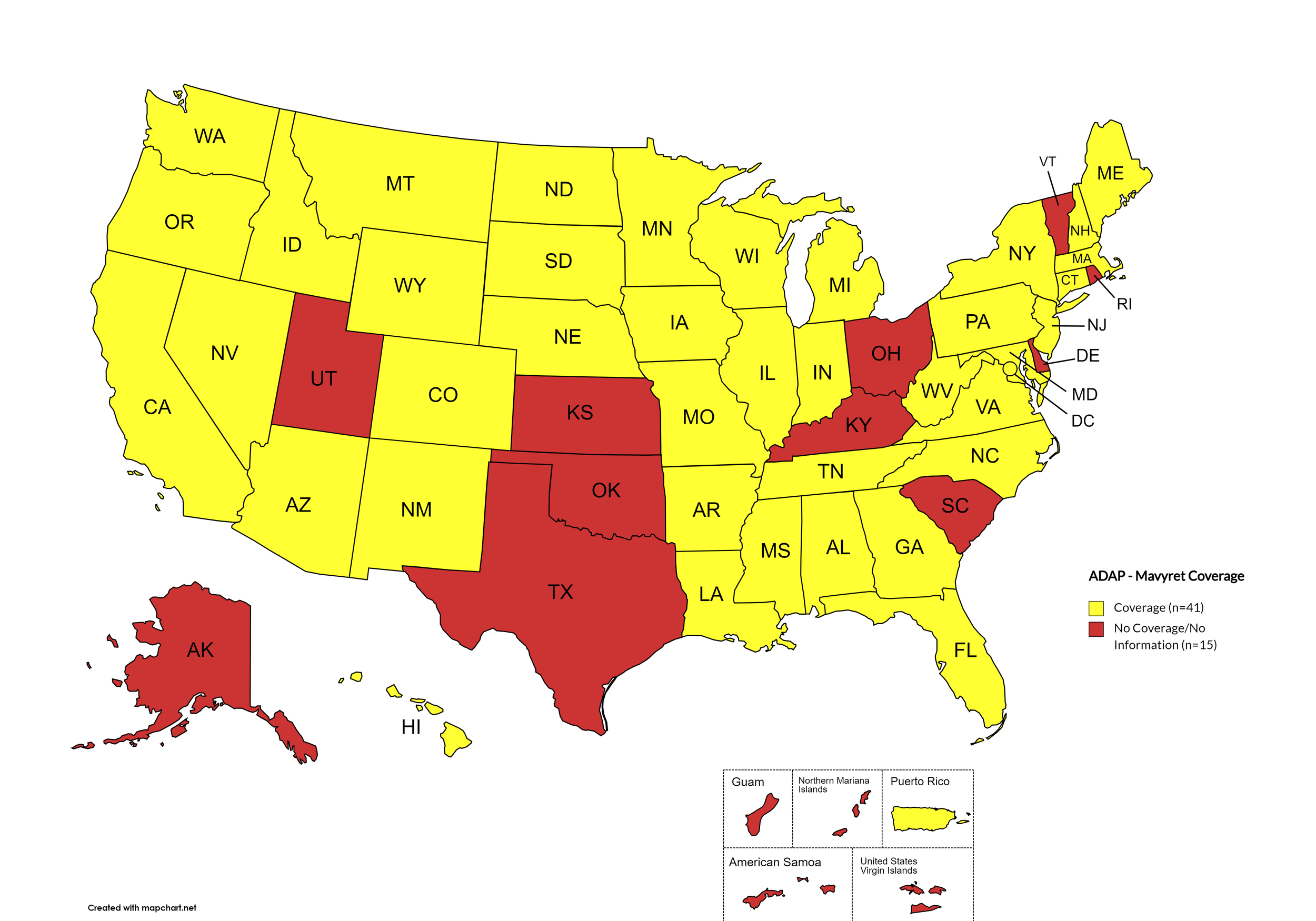
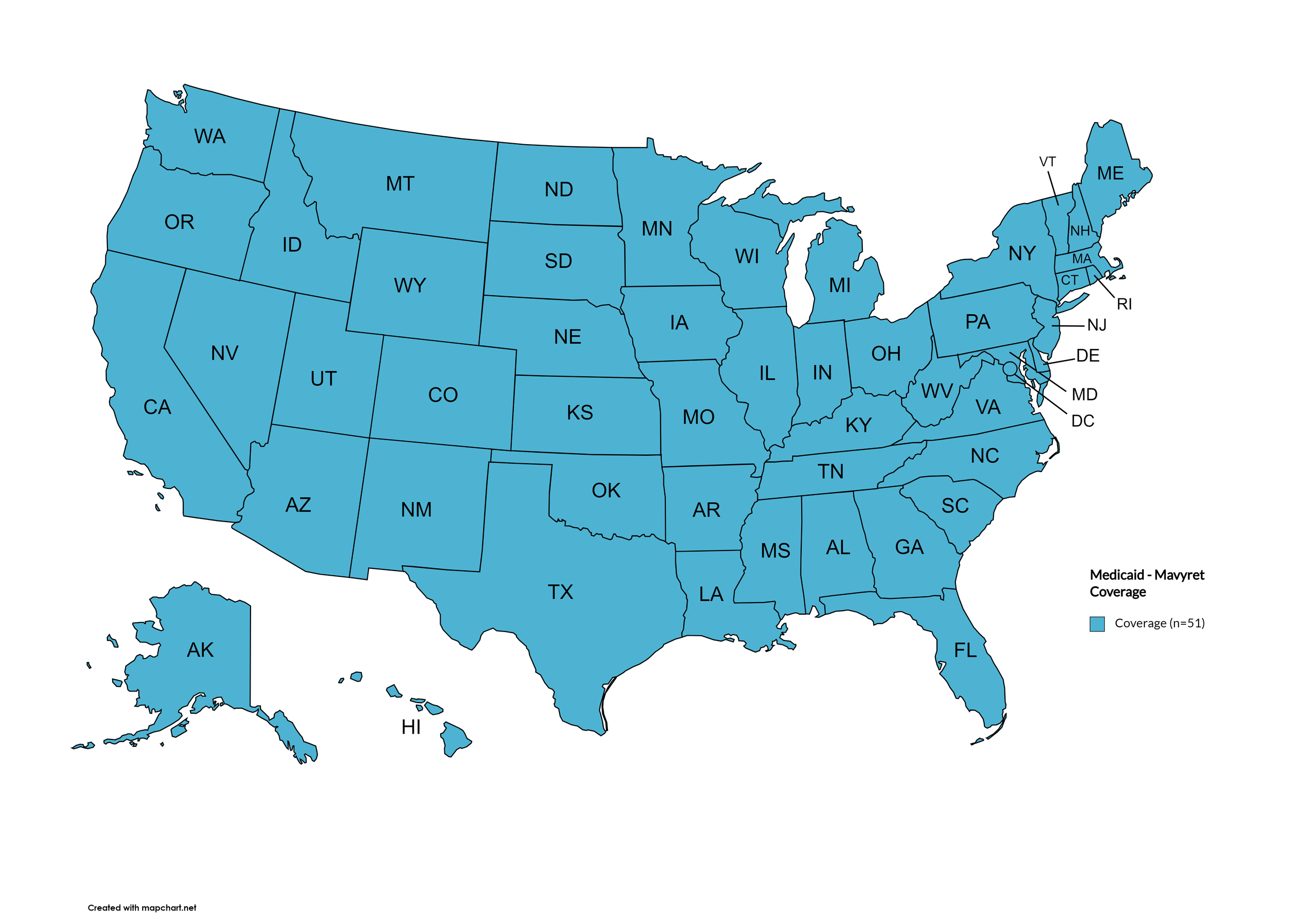
Mavyret
States with Mavyret Coverage: AL, AZ, AR, CA, CO, CT, FL, GA, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OR, PA, SD, TN, VA, WA, WV, WI, WY, D.C.
States without Mavyret Coverage: AK, DE, KS, KY, OH, OK, RI, SC, TX, UT, VT
Territories with Mavyret Coverage: P.R.
Figure 7. April 2024 ADAP Coverage - Mavyret
Map Key: Yellow = Mavyret Coverage; Red = No Mavyret Coverage/No Information regarding Mavyret Coverage
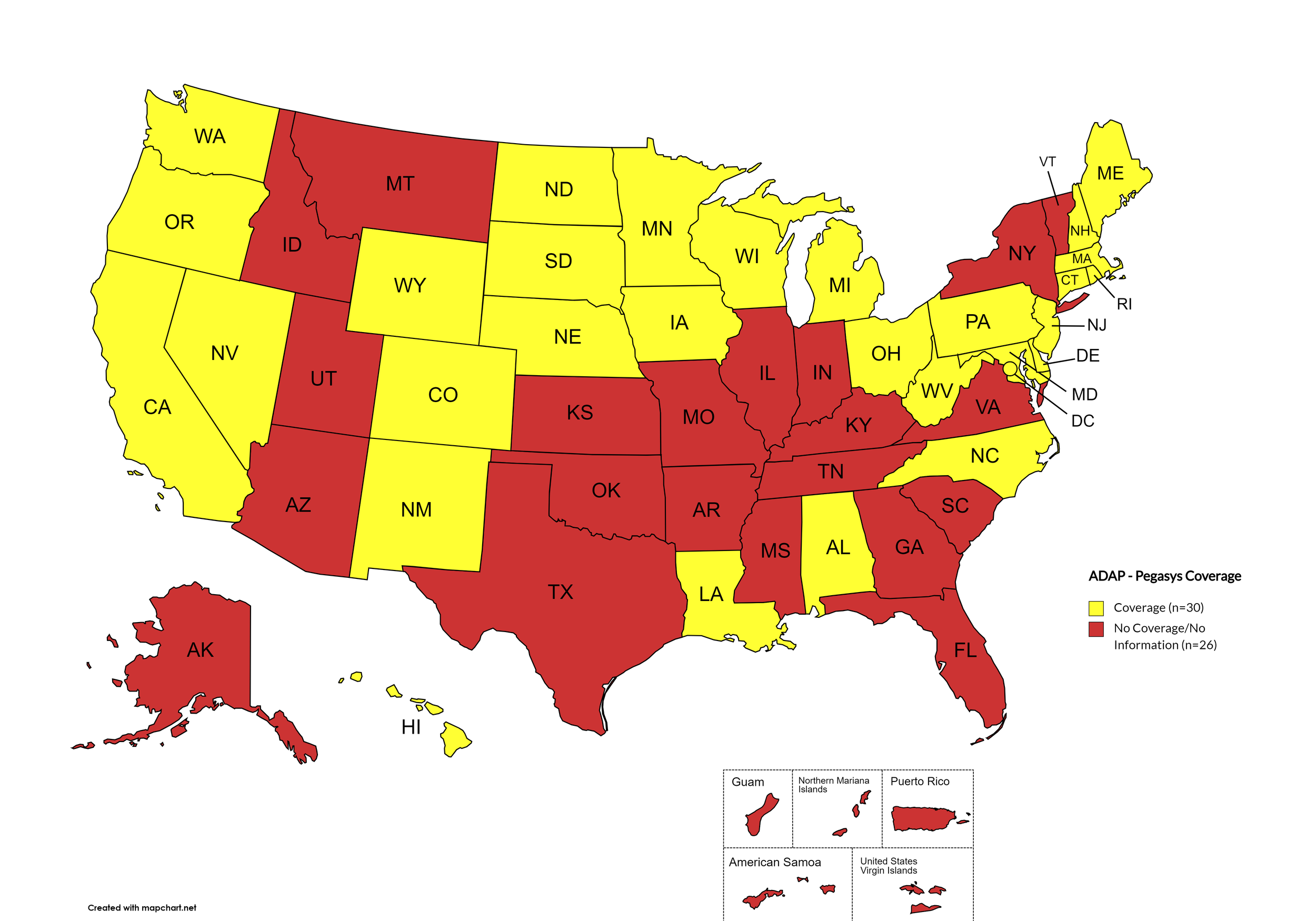
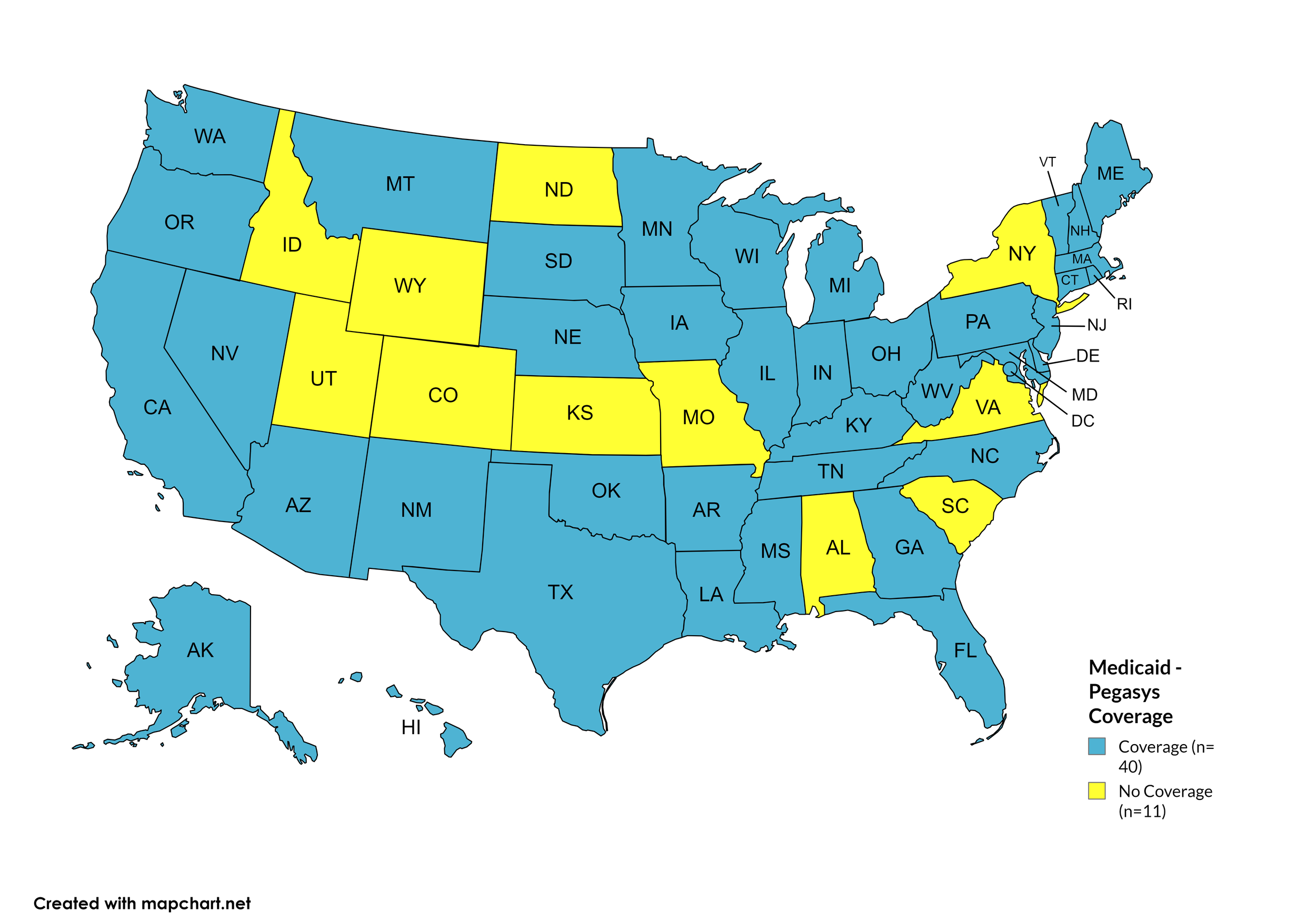
Pegasys
States with Pegasys Coverage: AL, CA, CO, CT, DE, HI, IA, LA, ME, MD, MA, MI, MN, NE, NV, NH, NJ, NM, NC, ND, OH, OR, PA, RI, SD, WA, WV, WI, WY, D.C.
States without Pegasys Coverage: AK, AZ, AR, FL, GA, ID, IN, KS, KY, MS, MO, MT, NY, OH, OK, SC, TN, TX, UT, VT, VA
Territories with Pegasys Coverage: None/Unknown
Figure 8. April 2024 ADAP Coverage - Pegasys
Map Key: Yellow = Pegasys Coverage; Red = No Pegasys Coverage/No Information regarding Pegasys Coverage
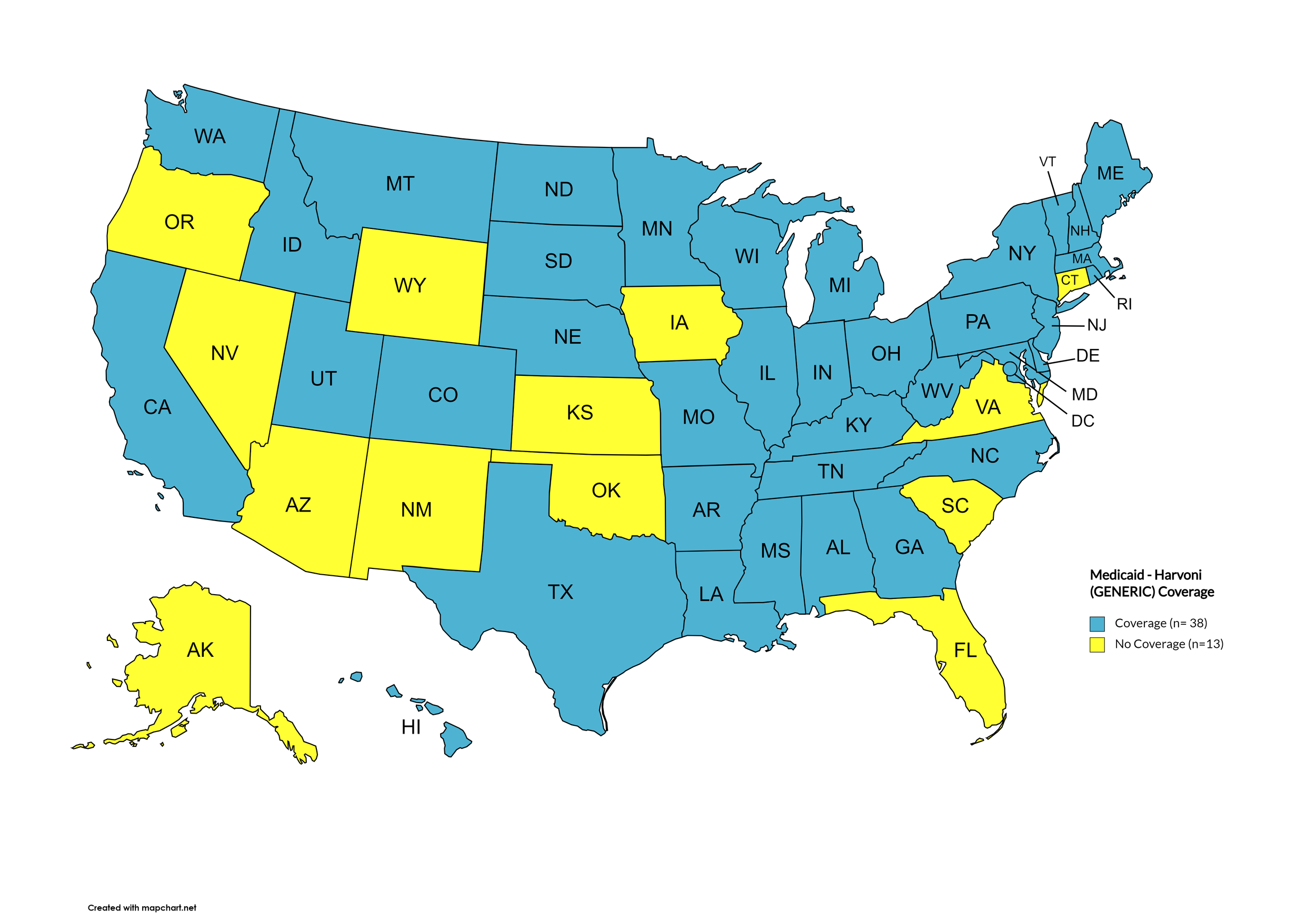
Harvoni (generic)
States with Harvoni (generic) Coverage: AR, CA, CO, CT, FL, GA, HI, IL, IA, ME, MD, MA, MN, NE, NV, NH, NJ, NM, NC, ND, OK, OR, PA, SD, TN, WA, WI, WY, D.C.
States without Harvoni (generic)Coverage: AL, AK, AZ, DE, ID, IN, KS, KY, LA, MI, MS, MO, MT, NY, OH, RI, SC, TX, UT, VT, VA, WV
Territories with Harvoni (generic) Coverage: P.R.
Figure 9. April 2024 ADAP Coverage - Harvoni (Generic)
Map Key: Yellow = Harvoni (Generic) Coverage; Red = No Harvoni (Generic) Coverage/No Information regarding Harvoni (Generic) Coverage
Epclusa (generic)
States with Epclusa (generic) Coverage: AR, CA, CO, CT, FL, GA, HI, IL, IA, ME, MD, MA, MN, NE, NV, NH, NJ, NM, ND, OR, PA, SD, TN, WA, WI, WY
States without Epclusa (generic) Coverage: AL, AK, AZ, DE, ID, IN, KS, KY, LA, MS, MO, MI, MT, NY, NC, OH, OK, RI, SC, TX, UT, VT, VA, WV, D.C.
Territories with Epclusa (generic) Coverage: P.R.
Figure 10. April 2024 ADAP Coverage - Epclusa (generic)
Map Key: Yellow = Epclusa (generic) Coverage; Red = No Epclusa (generic) Coverage/No Information regarding Epclusa (generic) Coverage
April 2024 Notes:
States with Open Formularies: IL, IA, MA, MN, NE, NH, NJ, NM, ND, OH, OR, WA, WY
N.B. – Although Ohio is listed by NASTAD as having an open formulary, both NASTAD’s ADAP Formulary Database and Ohio’s ADAP website indicates that the state does not offer any treatment for HCV.
N.B. – Although North Dakota has adopted an open formulary, they provide only co-pay and deductible assistance for HCV medications.
N.B. – Wyoming's ADAP Open Formulary document, the following disclaimer related to HCV is made: Hepatitis C treatment medications (i.e. Harvoni, Sovaldi, Ribavirin, Zepatier, Epclusa) must be prior authorized. To be eligible, clients must have applied for prior authorization from their insurance plan and the WY ADAP Hepatitis C Treatment checklist must be completed and signed by the provider and client.
California’s ADAP pharmacy benefit manager has recently removed coverage of ribavirin products for the treatment of HCV.
Colorado offers five coverage options – Standard ADAP, HIV Medical Assistance Program (HMAP), Bridging the Gap Colorado (BTGC), HIV Insurance Assistance Program (HIAP), and Supplemental Wrap Around Program (SWAP). ‘Yes’ indications in Figure 1. for Colorado denote that at least one of these programs offers coverage for each respective drug. The Standard ADAP Formulary covers medications only if funds are available to do so.
On August 11th, 2023, Georgia’s Department of Public Health issued a notice to Ryan White Part B District Coordinators, reading, in part, “Effective 8/14/2023, care providers will have the ability to order Hepatitis C medications for their eligible ADAP patients without the need for Prior Approval.” Initially covered medications are limited to ribavirn, Zepatier, Mavyret, and generics for Epclusa and Harvoni.
Hawaii’s ADAP notes the following: “Treatment slots for HCV direct-acting antivirals may be limited. Prescriber or pharmacy must call HDAP for slot.”
Louisiana’s ADAP (Louisiana Health Access Program – LA HAP) offers two coverage options – Uninsured (Louisiana Drug Assistance Program – L-DAP) and Insured (Health Insurance Program – HIP). HIP pays for the cost of treatment only if the client’s primary insurance covers the drug under its formulary.
Minnesota no longer covers Zapatier or Vosevi.
Mississippi’s ADAP formulary no longer covers generic Harvoni nor generic Epclusa.
Texas’s ADAP maintains no HCV coverage, despite a brief period of covering DAAs in 2022.
3. MEDICAID PROGRAMS & HCV THERAPIES
All 50 states and the District of Columbia continue to offer some form of HCV coverage. All 50 States and the District of Columbia have expanded their Preferred Drug Lists to include at least one HCV Direct Acting Agent (DAA).
April 2024 Updates:
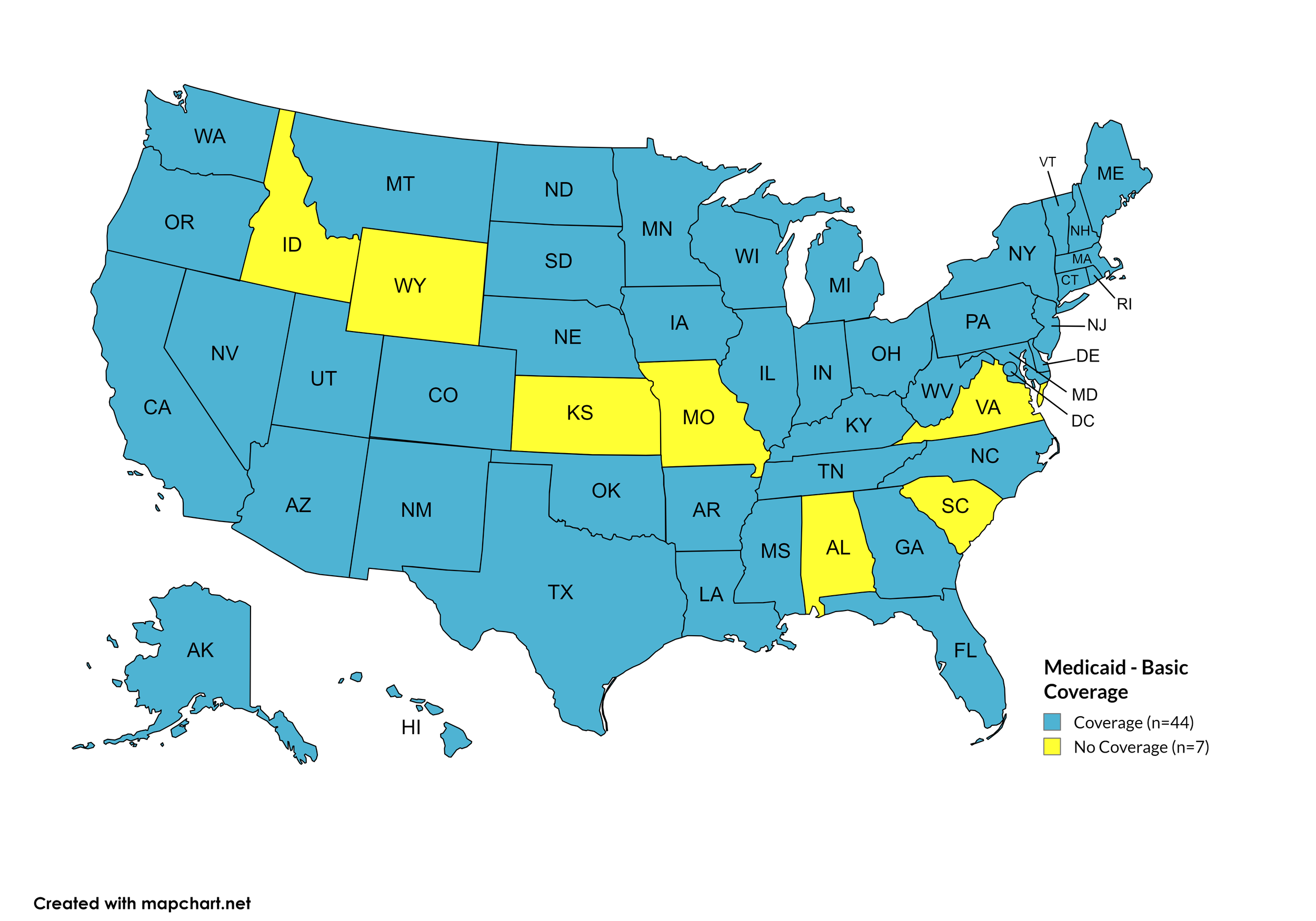
Basic Coverage
States with Basic HCV Medications Coverage: AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, WY, D.C.
States without Basic HCV Medications Coverage: AL, ID, KS, MO, SC, VA
Figure 11. April 2024 Medicaid Coverage - Basic HCV Medications
Map Key: Blue = Basic HCV Medication Coverage; Yellow = No Basic HCV Medication Coverage/No Information regarding Basic HCV Medication Coverage
Sovaldi
States with Sovaldi Coverage: AR, CA, CO, DE, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WI, D.C.
States without Sovaldi Coverage: AL, AK, AZ, CT, FL, IA, NH, NM, NV, OK, OR, SC, VA, WV, WY.
Figure 12. April 2024 Medicaid Coverage - Sovaldi
Map Key: Blue = Sovaldi Coverage; Yellow = No Sovaldi Coverage/No Information regarding Sovaldi Coverage
Harvoni
States with Harvoni Coverage: AL, AR, CA, CO, DE, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NH, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Harvoni Coverage: AK, AZ, CT, FL, IA, NV, NM, OK, OR, SC, VA, WY.
Figure 13. April 2024 Medicaid Coverage - Harvoni
Map Key: Blue = Harvoni Coverage; Yellow = No Harvoni Coverage/No Information regarding Harvoni Coverage
Zepatier
States with Zepatier Coverage: AL, AR, CA, CO, DE, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NE, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WI, D.C.
States without Zepatier Coverage: AK, AZ, CT, FL, IA, NV, NM, OK, OR, SC, VA, WV, WY.
Figure 14. April 2024 Medicaid Coverage - Zepatier
Map Key: Blue = Zepatier Coverage; Yellow = No Zepatier Coverage/No Information regarding Zepatier Coverage
Epclusa
States with Epclusa Coverage: AL, CA, CO, DE, GA, HI, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NH, NJ, NY, NC, ND, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Epclusa Coverage: AK, AZ, AR, CT, FL, ID, IA, NE, NV, NM, OH, OK, OR, SC, VA, WY.
Figure 15. April 2024 Medicaid Coverage - Epclusa
Map Key: Blue = Epclusa Coverage; Yellow = No Epclusa Coverage/No Information regarding Epclusa Coverage
Vosevi
States with Vosevi Coverage: AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NH, NJ, NY, NC, ND, OH, PA, RI, SC, SD, TN, TX, UT, VT, WA, WI, D.C.
States without Vosevi Coverage: AL, AK, AZ, IA, NV, NM, OK, OR, VA, WV, WY.
Figure 16. April 2024 Medicaid Coverage - Vosevi
Map Key: Blue = Vosevi Coverage; Yellow = No Vosevi Coverage/No Information regarding Vosevi Coverage
Mavyret
States with Mavyret Coverage: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
Figure 17. April 2024 Medicaid Coverage - Mavyret
Map Key: Blue = Mavyret Coverage; Yellow = No Mavyret Coverage/No Information regarding Mavyret Coverage
Pegasys
States with Pegasys Coverage: AK, AZ, AR, CA, CT, DE, FL, GA, HI, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MS, MT, NE, NV, NH, NJ, NM, NC, OH, OK, OR, PA, RI, SD, TN, TX, VT, WA, WV, WI, D.C.
States without Pegasys Coverage: AL, CO, ID, KS, MO, NY, ND, SC, UT, VA, WY
Figure 18. April 2024 Medicaid Coverage - Pegasys
Map Key: Blue = Pegasys Coverage; Yellow = No Pegasys Coverage/No Information regarding Pegasys Coverage
Harvoni (generic)
States with Harvoni (generic) Coverage: AL, AR, CA, CO, DE, GA, HI, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NH, NJ, NC, ND, OK, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Harvoni (generic) Coverage: AK, AZ, CT, FL, IA, KS, NM, NV, OK, OR, SC, VA, WY
Figure 19. April 2024 Medicaid Coverage - Harvoni (generic)
Map Key: Blue = Harvoni (generic) Coverage; Yellow = No Harvoni (generic) Coverage/No Information regarding Harvoni (generic) Coverage
Epclusa (generic)
States with Epclusa (generic) Coverage: AK, AL, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NJ, NM, NY, NC, ND, OH, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Epclusa (generic) Coverage: OK, NH
Figure 20. April 2024 Medicaid Coverage - Epclusa (generic)
Map Key: Blue = Epclusa (generic) Coverage; Yellow = No Epclusa (generic) Coverage/No Information regarding Epclusa (generic) Coverage
April 2024 Notes:
The following states’ Medicaid programs offer multiple coverage plans for their respective Medicaid clients. The plan highlighted in bold typeface represents the most comprehensive plan with the most drugs covered in the respective state:
Hawaii – (1.) Advantage Plus; (2.) QUEST Integration
New Jersey – (1.) Aetna; (2.) AmeriGroup NJ; (3.) Horizon NJ Health; (4.) UnitedHealthcare of New Jersey; (5.) WellCare
New Mexico – (1.) BlueCross BlueShield of New Mexico; (2.) Presbyterian Centennial Care; (3) Western Sky Community Care
Kentucky has a Unified Medicaid Formulary
Louisiana has a Unified Medicaid Formulary
Ohio – Ohio has a Unified Medicaid Formulary that applies to all MCOs
No data has been made available by the Medicaid programs in the U.S. Territories.
New York no longer covers Pegasys.
Ohio no longer covers brand name Epclusa. It only covers the generic.
Oklahoma covers Ribavirin (“basic”) now.
*Medicaid coverage excludes patients from most drug manufacturer patient assistance programs (PAPs)
4. VETERANS PROGRAMS & HCV THERAPIES
The Veteran's Administration (VA) currently offers coverage for all HCV drugs. This is according to the most recent VA National Formulary, dated May 2021 (U.S. Dept. of V.A., 2021a). The VA Treatment Considerations and Choice of Regimen for HCV-Mono-Infected and HIV/HCV Co-Infected Patients, dated March 2021 (U.S. Dept. of V.A., 2021b) lists the following therapies as preferred treatments:
Abbreviations:
- CTP – Child-Turcotte-Pugh (score used to assess severity of cirrhosis)
- IU/mL – International Units Per Milliliter
- PEG-IFN/IFN – Peginterferon/Interferon
- RAS – Resistance-associated substitutions
Genotype 1:
Treatment-naïve without or with cirrhosis (CTP A):
Pangenotypic regimens
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens:
Zepatier: 1 tablet orally daily for 12 weeks if GT1a without baseline NS5A RAS or GT1b
Harvoni: 1 tablet orally daily
If HCV-noninfected, non-cirrhotic, and HCV RNA baseline <6 million IU/mL: 8 weeks
If cirrhotic, baseline HCV RNA ≥6 million IU/mL, HIV/HCV-co-infected, or African American: 12 weeks
Consider adding ribavirin in CTP A patients
Treatment-naïve with decompensated cirrhosis (CTP B or C):
Harvoni: 1 tablet orally daily + ribavirin (600 mg/day and increase by 200 mg/day every 2 weeks only as tolerated) for 12 weeks
Epclusa: 1 tablet orally daily + ribavirin (1000 mg/day - <75kg – or 1,200 mg daily - ≥75kg – orally daily in 2 divided doses with food) for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb).
Treatment-experienced (NS5A- and SOF-naïve [e.g., failed PEG-IFN/RBV ± NS3/4A PI]) without or with cirrhosis (CTP A)
Pangenotypic regimens:
Mavyret: 3 tablets orally daily with food
If PEG-IFN/RBV-experienced: 8 weeks if non-cirrhotic or 12 weeks if cirrhotic
If NS3/4A PI + PEG-IFN/RBV-experienced: 12 weeks
Vosevi: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens
Zepatier: 1 tablet orally daily for 12 weeks if GT1b, or if failed only PEG-IFN/RBV and GT1a without baseline NS5A RAS
Harvoni: 1 tablet orally daily for 12 weeks
Treatment-experienced (NS5A-naïve and SOF-experienced) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food
If PEG-IFN/RBV + Sovaldi-experienced: 8 weeks if non-cirrhotic or 12 weeks if cirrhotic
If Olysio + Sovaldi-experienced: 12 weeks
Epclusa: 1 tablet orally daily for 12 weeks if GT1b
Vosevi: 1 tablet orally daily with food for 12 weeks if GT1a
Treatment-experienced (prior NS5A-containing regimen) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 16 weeks if failed only an NS5A inhibitor without NS3/4A PI (e.g., Harvoni)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-experienced with decompensated cirrhosis (CTP B or C)
Epclusa: 1 tablet orally daily + RBV; start at lower RBV doses as clinically indicated (e.g., baseline Hgb);
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks; NOT FDA approved for 24 weeks
Genotype 2:
Treatment-naïve or treatment-experienced (PEG-IFN/IFN ± RBV or Sovaldi + RBV ± PEG-IFN) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks; 12 weeks if CTP A and treatment-experienced or in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-naïve or treatment-experienced patients with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks
Genotype 3:
Treatment-naïve without cirrhosis or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks if cirrhotic or in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
If CTP A, test for NS5A RAS
Add ribavirin if Y93H RAS present
Treatment-experienced (PEG-IFN ± RBV or Sovaldi + RBV ± PEG-IFN) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 16 weeks
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
If CTP A, consider adding ribavirin (no supporting data)
Treatment-naïve or treatment-experienced with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks
Genotype 4:
Treatment-naïve without or with cirrhosis (CTP A)
Pangenotypic regimens
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens
Zepatier: 1 tablet orally daily for 12 weeks
Harvoni: 1 tablet orally daily for 12 weeks
Treatment-naïve with decompensated cirrhosis (CTP B or C)
Pangenotypic regimen
Epclusa: 1 tablet orally daily + RBV for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
Non-pangenotypic regimen:
Harvoni: 1 tablet orally daily + ribavirin (600 mg/day and increase by 200 mg/day every 2 weeks only as tolerated) for 12 weeks
Treatment-experienced (Sovaldi-experienced and NS5A-naïve) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks if NS3/4A PI-naïve without cirrhosis, and 12 weeks if NS3/4A PI-experienced or CTP A
Epclusa: 1 tablet orally daily + ribavirin for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-experienced with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks; NOT FDA approved for 24 weeks
5. PATIENT ASSISTANCE PROGRAMS
The drug manufacturers and various national nonprofit organizations offer a variation of patient assistance programs (PAPs) to assist patients in accessing treatments. They include:
Support Path (Gilead Sciences):
Financial Assistance
Provides Co-Pay Coupons for Sovaldi, Harvoni, Harvoni (Generic), Epclusa, Epclusa (Generic), and Vosevi
Co-Pay Coupons cover out-of-pocket costs up to 25% of the catalog price of a 12-week regimen (3 bottles/packages) of Sovaldi, Harvoni, Harvoni (Generic), Epclusa, Epclusa (Generic), or Vosevi
Excludes patients enrolled in Medicare Part D or Medicaid
Insurance Support
Researches and verifies patient’s benefits, and gives information they need about coverage options and policies
Explain Prior Authorization process and works with HCV Specialist’s office so they can submit PA forms to a patient’s insurance company
May be able to provide assistance with appeals process
Website: http://www.mysupportpath.com/
AbbVie Mavyret Co-Pay Savings Card:
Financial Assistance
Patient may be eligible to pay as little as $5
Excludes patients enrolled in Medicare Part D, Medicare Advantage, Medigap, Medicaid, TRICARE, Department of Defense, or Veterans Affairs programs)
NeedyMeds:
NeedyMeds Drug Discount Card
Designed to lower cost of prescription medications by up to 80% at participating pharmacies
Price finder tool for the drug discount card
No eligibility requirements
CANNOT be used in combination with government healthcare programs, but CAN be used IN PLACE of program
CANNOT be combined with other offers
Website: http://ow.ly/fEJo309cJ7Z
The Assistance Fund:
Status: WAITLISTED
Requires provider referral
Copay assistance
Eligibility Criteria:
US citizen or permanent resident
Diagnosed with the disease for which you are applying
Prescribed an FDA-approved treatment for the disease
Have prescription coverage for the prescribed treatment
Meet financial eligibility criteria based upon household income and size
Patient Advocate Foundation Co-Pay Relief:
Status: CLOSED
Maximum award of $15,000
Eligibility Requirements:
Patient must be insured, and insurance must cover prescribed medication
Confirmed HCV diagnosis
Reside and receive treatment in the U.S.
Income falls below 400% of FPL with consideration of the Cost of Living Index (COLI) and the number in the household
Patient Access Network (PAN) Foundation:
Status: OPEN
Co-Pay Assistance with a maximum award of $5,600
Patients may apply for additional assistance during their eligibility period, subject to availability of funding
Eligibility Requirements:
Must be getting treatment for HCV
Have insurance that covers prescribed HCV medication
Medication must be listed on PAN’s list of covered medications: https://www.panfoundation.org/index.php/en/patients/medications-covered
Income falls below 500% of FPL
Residing and receiving treatment in the U.S. (citizenship NOT required)
Website: https://www.panfoundation.org/index.php/en/patients/assistance-programs/hepatitis-c
HealthWell Foundation:
Status: OPEN
Co-Pay Assistance with a maximum award of $30,000
Minimum Co-Pay Reimbursement Amount: None
Minimum Premium Reimbursement Amount: None
Eligibility Requirements:
Must be being treated for HCV
Have insurance that covers HCV prescribed medication
Income falls below 500% of FPL
Receiving treatment in the U.S.
Website: https://www.healthwellfoundation.org/fund/hepatitis-c/
6. HARM REDUCTION PROGRAMS
Harm Reduction, as it relates to opioid abuse and HCV, are measures designed to serve as preventive or monitoring efforts in combating opioid prescription drug and heroin abuse, and as an effect, helping to prevent the spread of HCV and HIV. The Co-Infection Watch covers the following measures: Syringe Exchange, Expanded Naloxone Access, State Authorized Safe Consumption Sites, Updated Paraphernalia Laws (allowing for possession of substance testing strips), Good Samaritan Laws, Required Prescriber Education. (Editor’s Note: Program descriptions provided herein).
April 2024 Updates:
Syringe Exchange
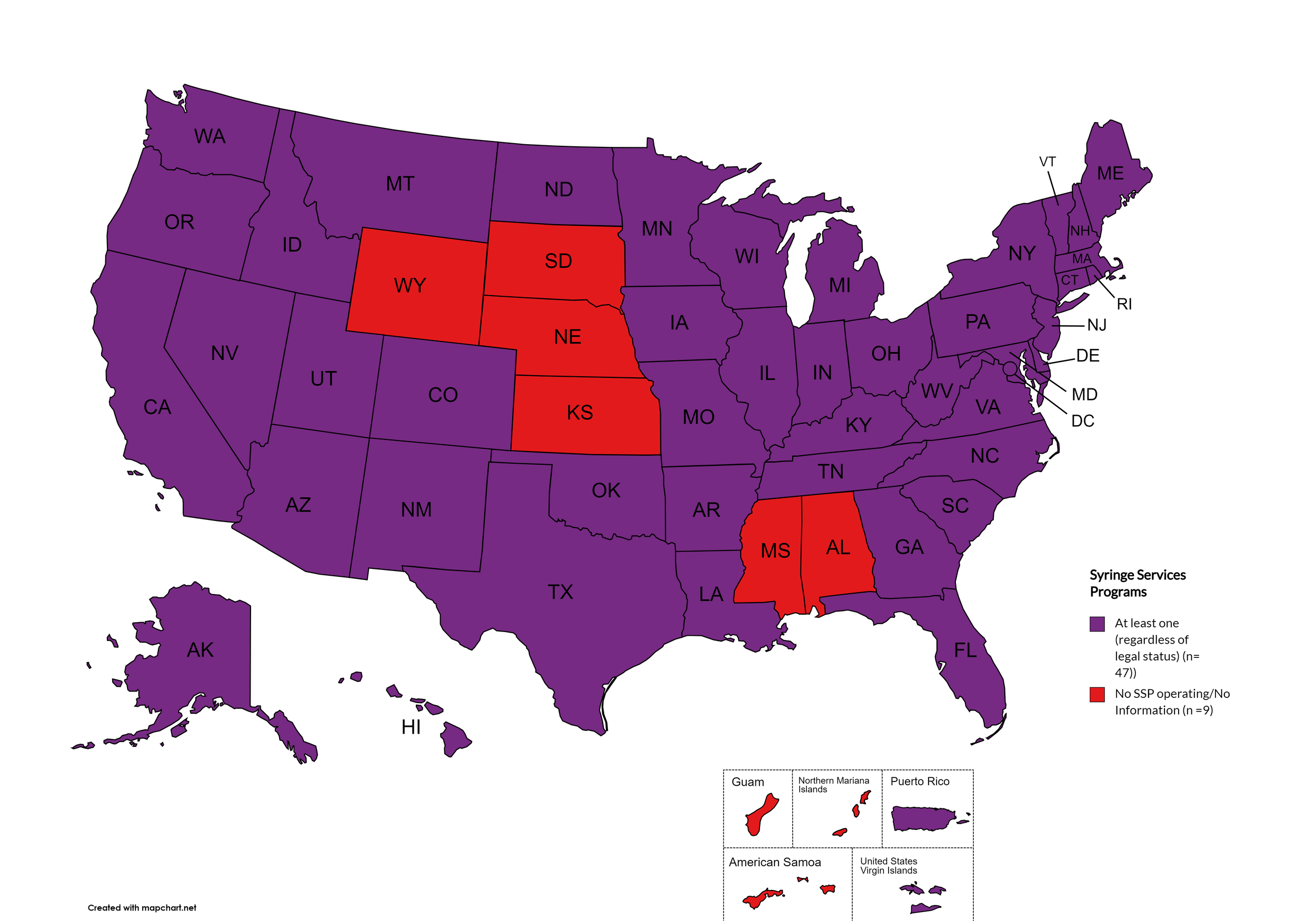
Syringe Services Programs (SSPs) exist to provide injection drug users (or those whose prescriptions require injection) with clean syringes and/or in exchange for used ones. (N.b. – states listed as "at least one SSP…” indicate only that a Syringe Services Program (SSP) exists within the state, regardless of the legality of SSPs under state law).
States with Syringe Exchange: AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MO, MT, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, D.C.
States without Syringe Exchange: AL, KS, MS, NE, SD, WY
Territories with Syringe Exchange: Puerto Rico, U.S. Virgin Islands
Figure 21. April 2024 Syringe Exchange Coverage
Map Key: Purple = Syringe Exchange(s); Red = No Syringe Exchange(s); Grey = No Information
Expanded Naloxone
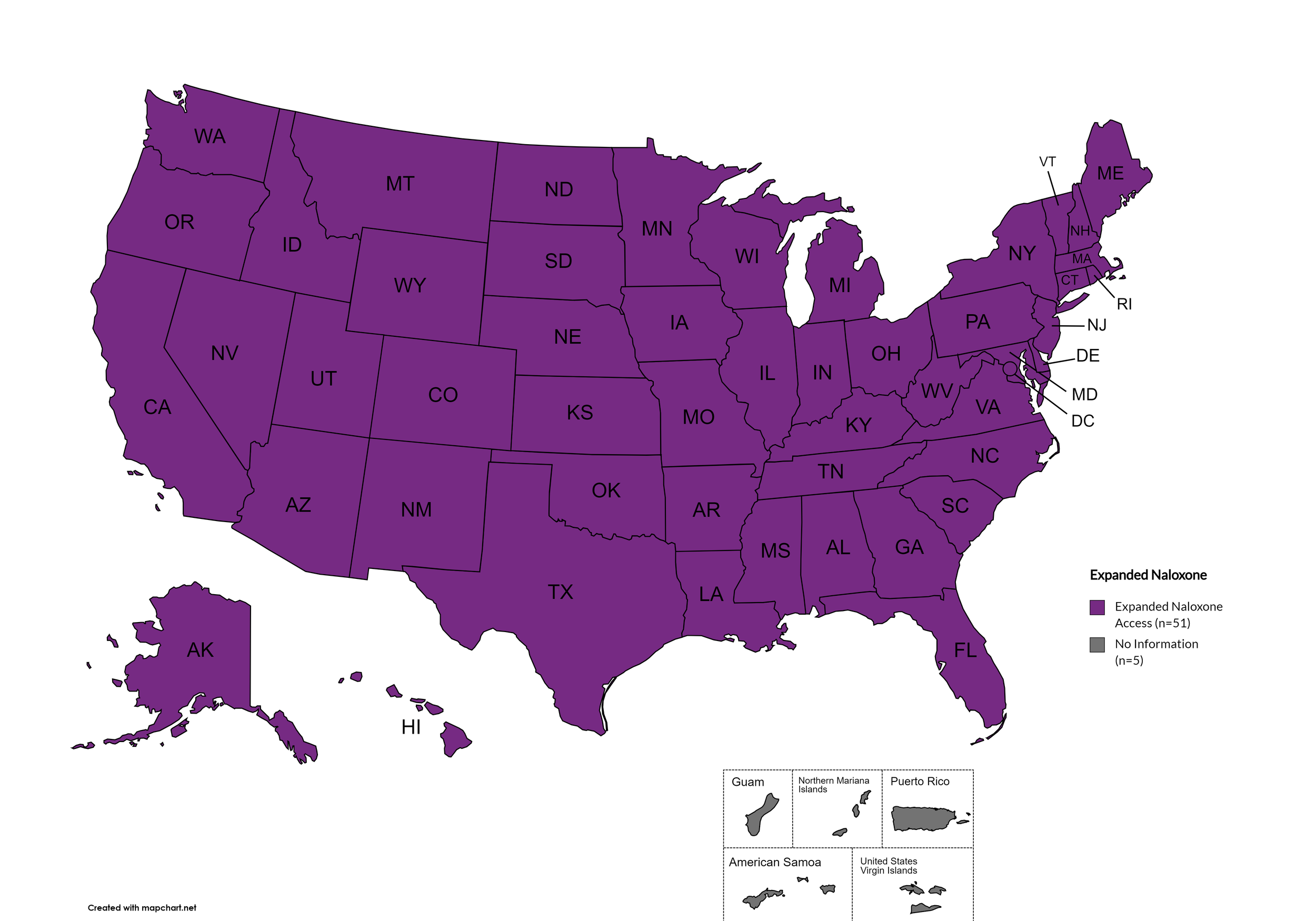
Naloxone is a drug used to counteract the effects of opioid overdoses. Expanded Access refers to having statutes or state standing orders in place that allow pharmacies to dispense naloxone without a prescription. This means those in danger of overdose, those who are caregivers for them, or anyone who may come in contact with those in danger of overdose can walk into a pharmacy and obtain naloxone without a prescription. Removing the requirement of a patient-doctor relationship via prescription enhances access.
States with Expanded Naloxone: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Expanded Naloxone: None
Territories with Expanded Naloxone: Unknown
Figure 22. April 2024 Expanded Naloxone Coverage
Map Key: Purple = Expanded Naloxone; Red = Restricted Naloxone; Gray = No Information
State Authorized Safe Consumption Sites
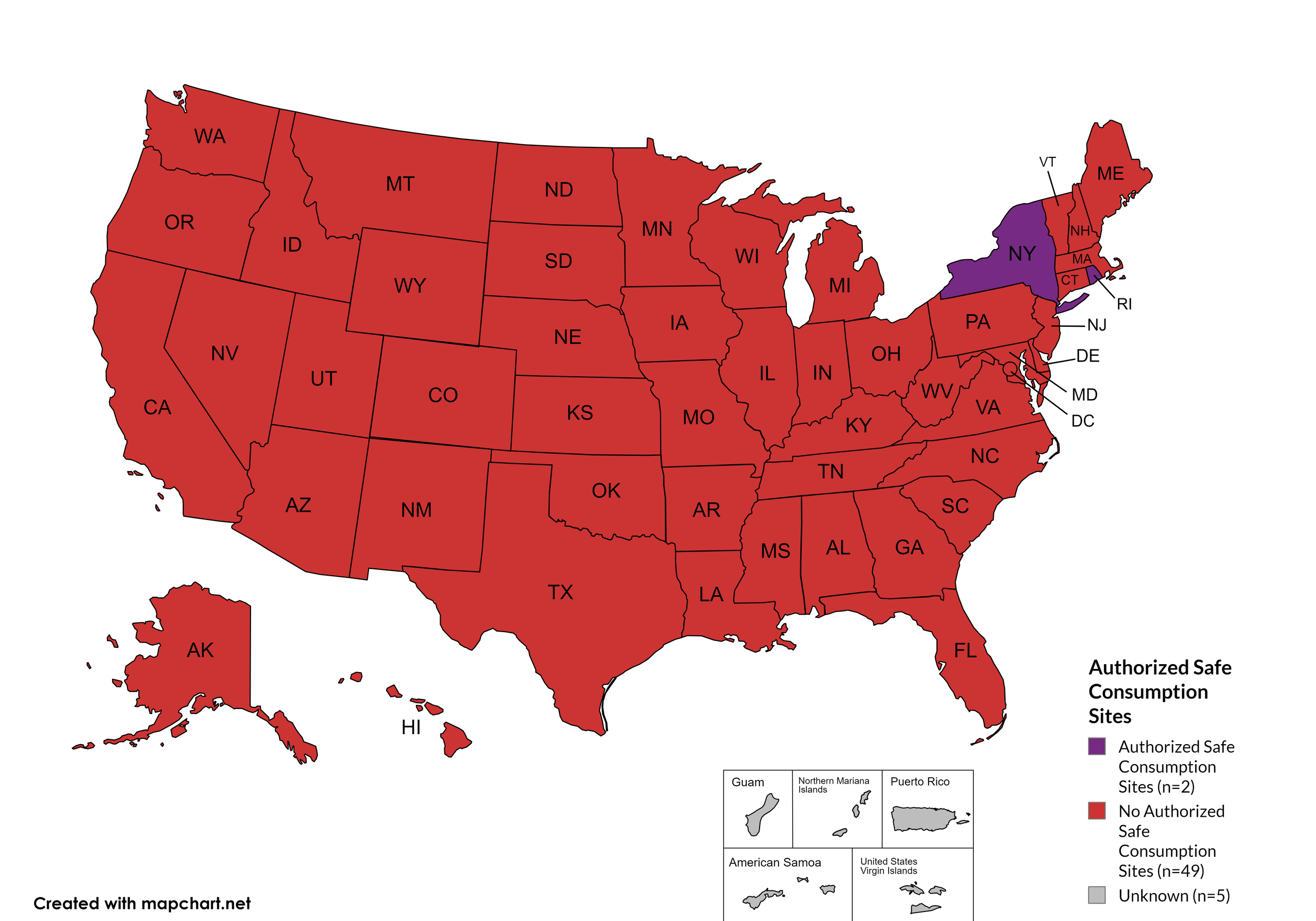
Federal prohibits the distribution, possession, and consumption of certain controlled substances. Safe Consumption Sites (SCSs) exist to provide injection drug users (or those whose prescriptions require injection) with clean syringes and/or in exchange for used ones, offer wound care supplies, allow for injection drugs users to consume drugs, offer infectious disease screening, and other linkage to care opportunities. This section monitors state authorized safe consumption site programs and pilot projects related to safe consumption sites.
States with Safe Consumption Sites: NY, RI
States without Safe Consumption Sites: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NC, ND, OH, OK, OR, PA, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
Territories with Safe Consumption Sites: None
Figure 23. April 2024 State Authorized Safe Consumption Sites
Map Key: Purple = States with sites; Red = States without sites
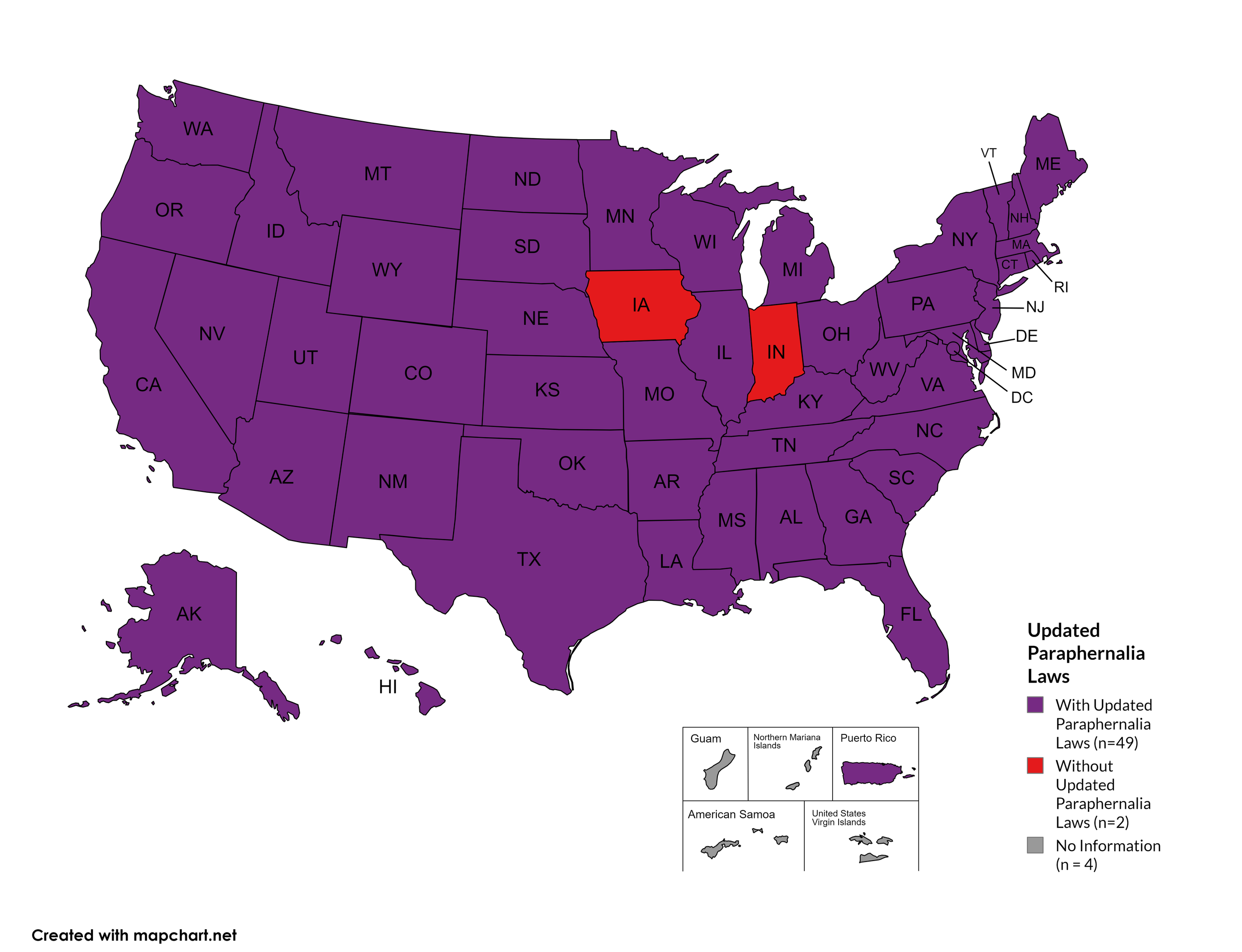
Updated Paraphernalia Laws
State paraphernalia laws have long prohibited possession of certain drugs use related materials, including harm reduction materials like fentanyl testing strips. Some states have modernized their criminal codes to allow for possession of testing strips and may also have health department programs distributing testing strips.
States with Updated Paraphernalia Laws: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, KS, KY, LA, MD, MA, ME, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Updated Paraphernalia Laws: IN, IA
Territories with Updated Paraphernalia Laws: Unknown
Figure 24. April 2024 Updated Paraphernalia laws
Map Key: Purple = Without Updated Laws; Red = With Updated Laws; Gray = No Information
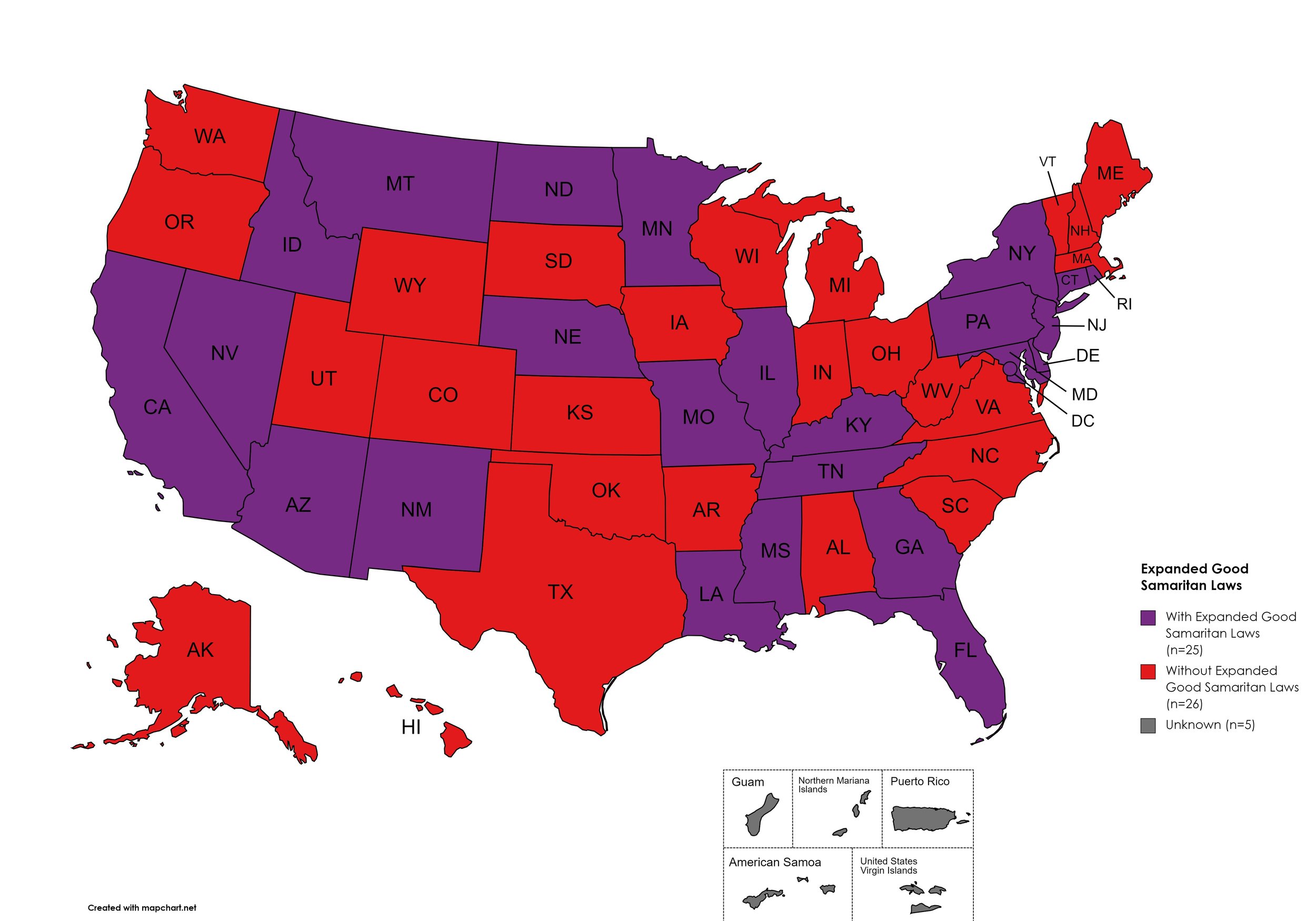
Expanded Good Samaritan Laws
Expanded Good Samaritan Laws are laws that are designed to protect persons seeking emergency services for drug overdoses from drug-related charges or prosecutions, regardless of possession or consumption of illegal, illicit substances, or drug paraphernalia. Good Samaritan laws may or may not provide protection to those currently under parole or probation. Good Samaritan laws listed do NOT prohibit arrest.
States with Expanded Good Samaritan Laws: AZ, CA, CT, DE, Fl, GA, HI, ID, IL, KY, LA, MD, MN, MS, MO, MT, NB, NV NJ, NM, NY, ND, PA, RI, TN
States without Expanded Good Samaritan Laws: AL, AK, AR, CO, IN, IA, KS, ME, MA, MI, NH, NC, OH, OK, OR, SC, SD, TX, UT, VT, VA, WV, WI, WY
Territories with Expanded Good Samaritan Laws: Unknown
Figure 25. April 2024 Good Samaritan Laws Coverage
Map Key: Purple = Good Samaritan Laws; Red = No Good Samaritan Laws; Gray: No Information
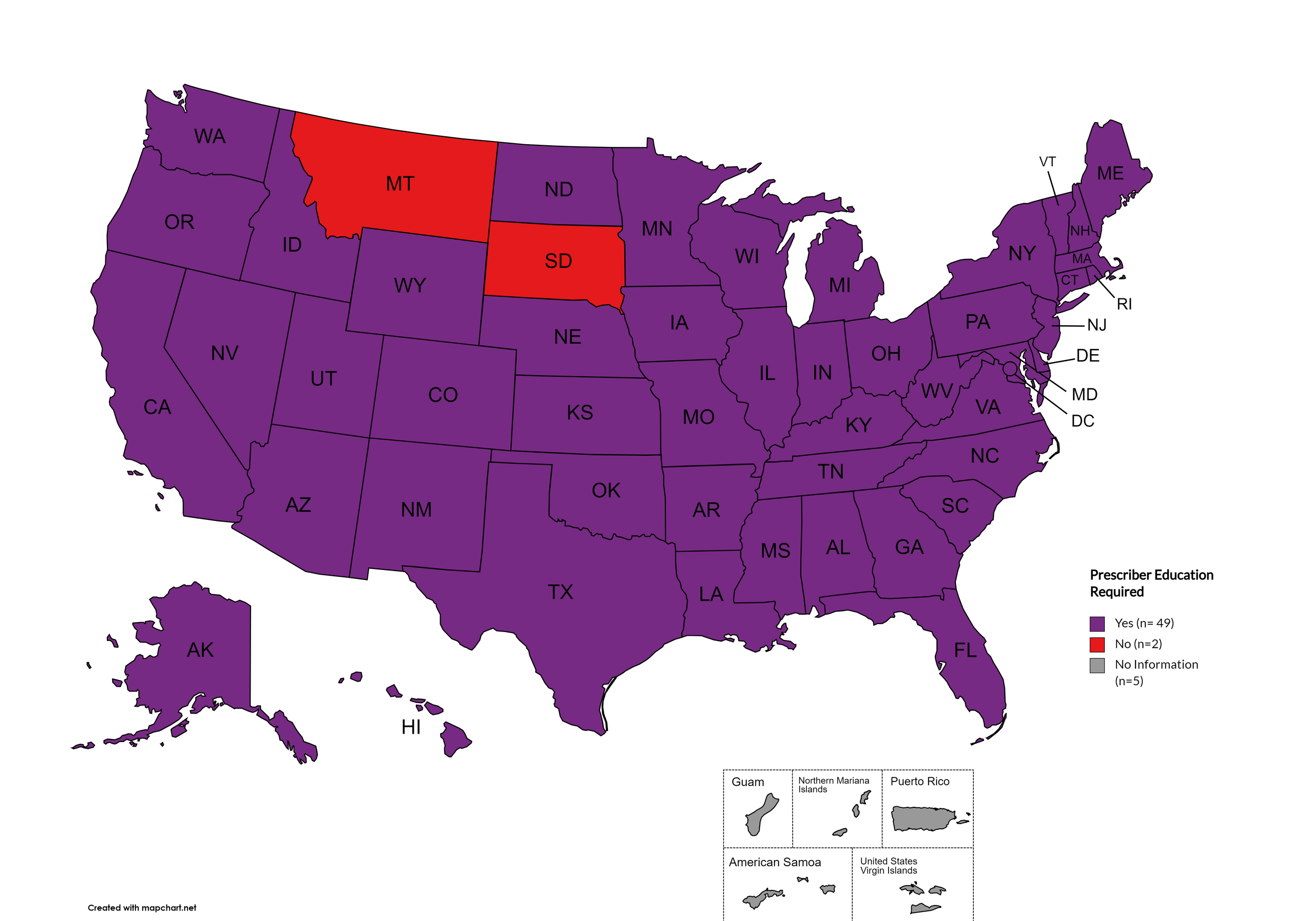
Prescriber Education Required
States that require/do not require through legislative action or regulatory or licensing bodies that prescribing physicians undergo special training in addition to or as part of their initial education to become prescribers related to safer controlled substance and/or pain management prescribing and utilization practices.
States with Prescriber Education Required: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MO, MN, MS, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Prescriber Education Required: MT, SD
Territories with Prescriber Education Required: Unknown
Figure 26. April 2024 Prescriber Education Required Coverage
Map Key: Purple = Prescriber Ed Required; Red = No Prescriber Ed Required; Gray = No Information
April 2024 Notes:
Metrics for Mandatory PDMP reporting, Doctor Shopping Laws, Physical Exam, ID Requirements, and Lock-in Pharmacy programs have been permanently deleted from the Watch due to redundancy or outdatedness.
Metric definition for Expanded Naloxone was updated. Medicaid covers naloxone in all states. However, even though naloxone was previously made available OTC federally, some states still required a prescription for it at the pharmacy. Expanded Naloxone designation also addresses issues such as its availability in schools and other public places, the state-by-state variation in who other than pharmacists is allowed to dispense naloxone, and more.
Added metrics for monitoring state paraphernalia laws regarding possession of testing strips and state authorized safe consumption sites, including legislatively authorized pilot projects.
NC, ND, and VT still have controlled substance testing equipment on their drug paraphernalia law statues but provide a carve-out to allow testing strips.
Metric definition for Prescriber Education has been updated to exclude “recommended” and only reflect those states which have laws or licensing board requirements of initial and/or continuing education for prescribers with regard to pain management and/or the prescription of controlled substances.
Some states have general requirements regarding “controlled substances”, some states are explicit with regard to category of controlled substance or type of controlled substance (ie. “opioids”).
This adjustment clarifies that MT and SD are the only states that do not require opioid specific and/or pain management specific and/or controlled substances prescribing education by law or licensing institution in either core or continuing education for providers.
This adjustment clarifies that KS, MO, and ND do require opioid specific and/or pain management specific and/or controlled substances prescribing education by law or licensing institution in either core or continuing education for providers.
The immunity provided under Good Samaritan Laws only applies when there are personal usage amounts of drugs present. It does not apply when there are distribution-level amounts.
In April, Idaho repealed its five-year-old Syringe and Needle Act by passing House Bill 617. The repeal awaits a veto or a signature by the governor. If he signs the bill, the repeal will become law and go into effect July 1st.
Indiana passed a bill to decriminalize fentanyl testing strips in the House. However, the bill died in the Senate.
Nebraska’s standing order for Naloxone is set to expire August of 2024.
Rhode Island approved the state’s first safe consumption site. It will open in Summer 2024 at 45 Willard Avenue, next to the Rhode Island Hospital Campus in Providence.
Wyoming law does not explicitly outlaw fentanyl testing strips, however they have not been widely distributed.
The DEA’s COVID-19-based waiver of in-person exams is expected to expire in November of 2023. A final rule on this change has not yet been published as of the time of this report. The DEA’s proposed rule was met with sufficient enough advocate, patient, and provider objection that it will not be taken up and a new proposed rule is being drafted as of the time of this writing. News: The DEA and HHS have again extended the full slate of tele-prescribing flexibilities allowed during the COVID-19 Public Health Emergency through December 31, 2024.
7. LATEST NEWS
Over half of state Medicaid programs have ended prior authorization requirement for first-time hepatitis C treatment - The Center for Health Law and Policy Innovation of Harvard Law School (CHLPI) and the National Viral Hepatitis Roundtable (NVHR) reported good news in their 2024 National Snapshot report. Twenty-eight jurisdictions now no longer require prior authorization for first-time treatment. States are being encouraged to look into their records and reach out to Medicaid patients over the past ten years who missed out on treatment due to prior authorization hurdles and are in need of antiviral medications to be cured. Along with the victory, work still needs to be done to ensure that the managed care organizations that run some states' Medicaid programs properly implement the changes.
Global increase in deaths from viral hepatitis - The World Health Organization (WHO) recently released a report at the World Hepatitis Summit in Lisbon, Portugal. The data from 187 countries show that deaths due to viral hepatitis are increasing. The data compares numbers from 2022 as compared to 2019. Approximately 17% of the deaths were caused by hepatitis C and 83% by hepatitis B. People 30-54 years old represent half the burden of chronic hepatitis B and C infections. Although there has been a slight decrease in the incidence of new infections, incidence levels are still high. In 2022, there were 2.2 million new infections, including 1 million new Hepatitis C infections and 1.2 million new hepatitis B infections.
Annual COVID-19 vaccine is a wise investment for personal health and individual finances - Because COVID-19 is no longer widely considered an exceptional public health risk, across the U.S. vaccination rates have dropped drastically. Businesses and other organizations are no longer mandating COVID-19 boosters, and the government is no longer subsidizing shots. To get boosters now people have to pay completely out of pocket or utilize their health insurance. Lowered vaccination coverage in tandem with waning infection-induced immunity could pose a challenge to the future transmission rates and severity of COVID-19. A recent Journal of Infectious Diseases study explored the clinical and financial benefits of an annual COVID-19 shot from an individual perspective as opposed to the customary payer perspective. The conclusion is that the annual COVID-19 vaccine is beneficial clinically and financially for individuals with and without insurance. The economic benefits were highest for those 50-64 years of age.
Ohio has six laws that penalize people living with HIV - Ohio currently has six antiquated laws on the books that penalize people living with HIV (PLWH). These laws either create harsher penalties for a crime if committed by PLWH or criminalize behaviors that are normally not criminal for the general population when involving PLWH. For example, spitting on someone would normally be met with a fine of up to $2500 or 6-12 months in imprisonment. If the person is HIV positive, the same offense is liable for a fine of up to $10,000 and 1-3 years in prison. This distinction falls under O.R.C 2921.38(C) Harassment with a bodily substance. The Felonious Assault law, O.RC. 2903.11(B) makes it a felony for PLWH to engage in sexual contact without disclosing their status, regardless of whether the person is undetectable, and can document their treatment. Additionally, they may be required to register as a sex offender. Advocates explain that in addition to the laws being unfair, they discourage people from HIV testing.
Scientists used gene therapy to “cut” HIV out of cells - A team of researchers at the University of Amsterdam recently reported a successful CRISPR experiment with HIV elimination. CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats. Simply put, CRISPR is molecular biological technology that utilizes enzymes and proteins to modify genes. In isolated cell culture, scientists were able to successfully seek out and ‘cut’ the genetic coding for HIV from cells leaving HIV inactivated and the cells ‘HIV-free.’ While the proof of concept is promising, science is many years away from utilizing CRIPSR as a tool to eradicate the HIV virus.
CMS extends special enrollment period for people no longer eligible for Medicaid, CHIP - The Medicaid continuous enrollment provision that existed during the declared Pandemic Health Emergency expired at the end of March 2023. Starting in April 2023, states were able to resume Medicaid disenrollments, which became known as the great ‘unwinding’. Millions are estimated to lose Medicaid coverage during the unwinding period. Some will lose coverage due to legitimate eligibility changes. Many will lose coverage due to flaws in the renewal system and states failing to handle the renewal process properly. The Centers for Medicare and Medicaid Services (CMS) extended a temporary special enrollment period (SEP) to help those losing Medicaid or CHIP eligibility to transition into Marketplace healthcare using HealthCare.gov. The CMS also released guidance to remind states of proper protocols for renewals since many people eligible for Medicaid and CHIP have lost coverage due to states improperly carrying out the process.
Research team reports on safe use of HIV and hepatitis C co-infected donor kidneys for transplant - There are over 88,000 people in the U.S. waiting for a kidney transplant. Increasing access to organs is in the best interests of public health since the number of people in need always outweighs the number of organs available. Virginia Commonwealth University Hume-Lee Transplant Center is researching transplanting kidneys from deceased donors who are co-infected with HIV and Hepatitis C into HIV-positive patients. Hume-Lee already researched and developed a protocol for transplanting kidneys infected with hepatitis C from a deceased donor into a patient without hepatitis. The process requires administering a course of antiviral medication before the transplant and has resulted in a 95% success rate of no hepatitis C appearing in the recipient. Gaurav Gupta, M.D., chair for the Division of Nephrology and Hume-Lee's co-medical director of kidney transplantation, and his team have presently performed three transplants on patients living with HIV receiving HIV and hepatitis C co-infected kidneys. The protocol involves a seven-day pre-transplant treatment of sofosbuvir and velpatasvir. The patients did not develop hepatitis C, and their HIV treatment was not adversely affected.