Watch 04: October 2023
The HIV/HCV Co-Infection Watch is a project of the Community Access National Network (CANN) designed to research, monitor and report on HIV and Hepatitis C (HCV) co-infection in the United States. The October 2023 Watch includes timely updates herein. To read the project disclaimer and/or methodology, CLICK HERE.
1. FINDINGS
The following is a summary of the key findings for October 2023:
AIDS Drug Assistance Programs:
There are 56 State and Territorial AIDS Drug Assistance Programs (ADAPs) in the United States, 47 of which offer some form of coverage for Hepatitis C (HCV) treatment. Of those programs, 45 have expanded their HCV coverage to include the Direct-Acting Antiviral (DAA) regimens that serve as the current Standard of Care (SOC) for Hepatitis C treatment. Two (2) programs offer only Basic Coverage and 9 programs offer No Coverage. One (1) program covers only a single Direct-Acting Antiviral. Three (3) territories – American Samoa, Marshall Islands, and Northern Mariana Islands – are not accounted for in this data. A state-by-state Drug Formulary breakdown of coverage is included in the October 2023 Updates, with accompanying drug-specific maps in Figures 1 – 10.
Medicaid Programs:
There are 59 State and Territorial Medicaid programs in the United States, and data is represented for all fifty (50) states and the District of Columbia. As of October 01, 2016, all 50 states and the District of Columbia offer Expanded Coverage. A state-by-state PDL breakdown of coverage is included in the October 2023 Updates, with accompanying drug-specific maps in Figures 11 – 20.
Harm Reduction Programs:
Every State and Territory in the United States currently provides funding for low-income people living with substance abuse issues to enter state-funded rehabilitation services (National Center for Biotechnology Information, n.d.). Forty-three (43) States, the District of Columbia and three (3) Territories currently have Syringe Services Programs (SSPs) in place, regardless of the legality. Fifty (50) States and the District of Columbia have expanded access to Naloxone to avert opioid drug overdoses. Fifty (50) States and the District of Columbia have Good Samaritan laws or statutes that provide some level of protection for those rendering emergency services during drug overdoses. Fifty (50) States, the District of Columbia, and Guam make reporting to Prescription Drug Monitoring Programs (PDMPs) mandatory, requiring physicians and/or pharmacists to report prescriptions written or filled to a state agency for monitoring. Fifty (50) States and the District of Columbia have Opioid-Specific Doctor Shopping Laws preventing patients from attempting to receive multiple prescriptions from numerous physicians, and/or from withholding information in order to receive prescriptions. Fifty (50) states and the District of Columbia mandate a Physical Exam Requirement in order for patients to receive a prescription for opioid drugs. Thirty-Five (35) states have in place an ID Requirement mandating that people filling opioid prescriptions present a state-issued ID prior to receiving their prescription. Forty-nine (49) states and the District of Columbia require prescribing physicians to attend mandatory and continuing opioid prescribing education sessions. Forty-seven (47) states and the District of Columbia have Medicaid doctor/pharmacy Lock-In programs that require patients to receive prescriptions from a single physician and/or fill prescriptions from a single pharmacy. A state-by-state program breakdown is included in the October 2023 Updates, with accompanying drug-specific maps in Figures 21-29.
2. AIDS DRUG ASSISTANCE PROGRAMS (ADAPs) & HCV THERAPIES
Of the 56 respective State and Territorial ADAPs, only 8 (KS, KY, OH, UT, VT, GU, PW, VI) do not offer any coverage for HCV drug therapies. States whose formularies are not available on the state-run website have been checked against the most recent National Alliance of State and Territorial AIDS Directors (NASTAD) formulary database (last updated January 1, 2023). The data presented are current as of October 22, 2023.
October 2023 Updates:
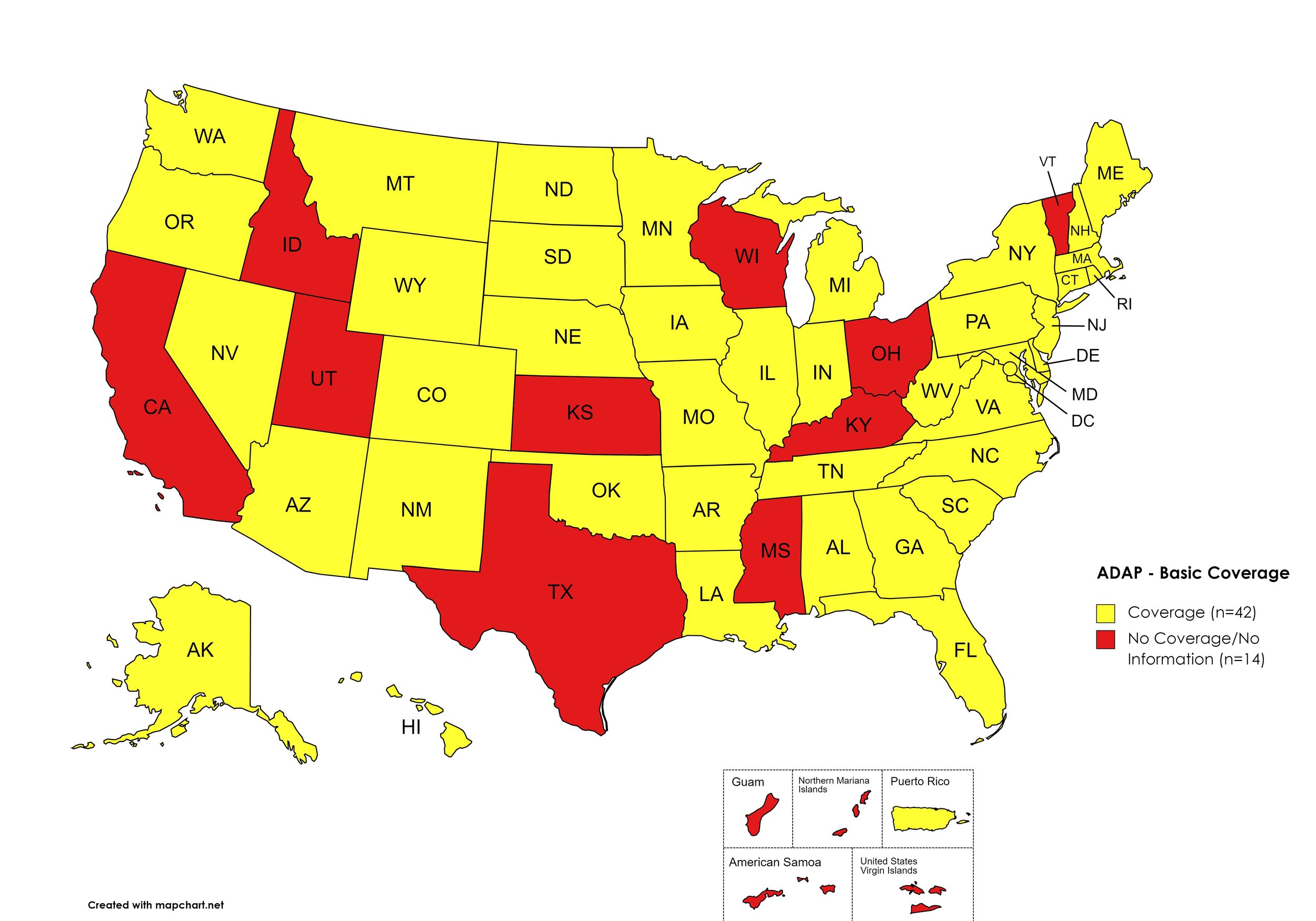
Basic Coverage
States with Basic HCV Medications Coverage: AL, AK, AZ, AR, CO, CT, DE, FL, GA, HI, IL, IN, IA, LA, ME, MD, MA, MI, MN, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OK, OR, PA, RI, SC, SD, TN, VA, WA, WV, WI, WY, D.C.
States without Basic HCV Medications Coverage: CA, ID, KS, KY, MS, OH, TX, UT, VT
Territories with Basic HCV Medications Coverage: P.R.
Figure 1. October 2023 ADAP Coverage - Basic
Map Key: Yellow = Basic Coverage; Red = No Basic Coverage/No Information regarding Basic Coverage
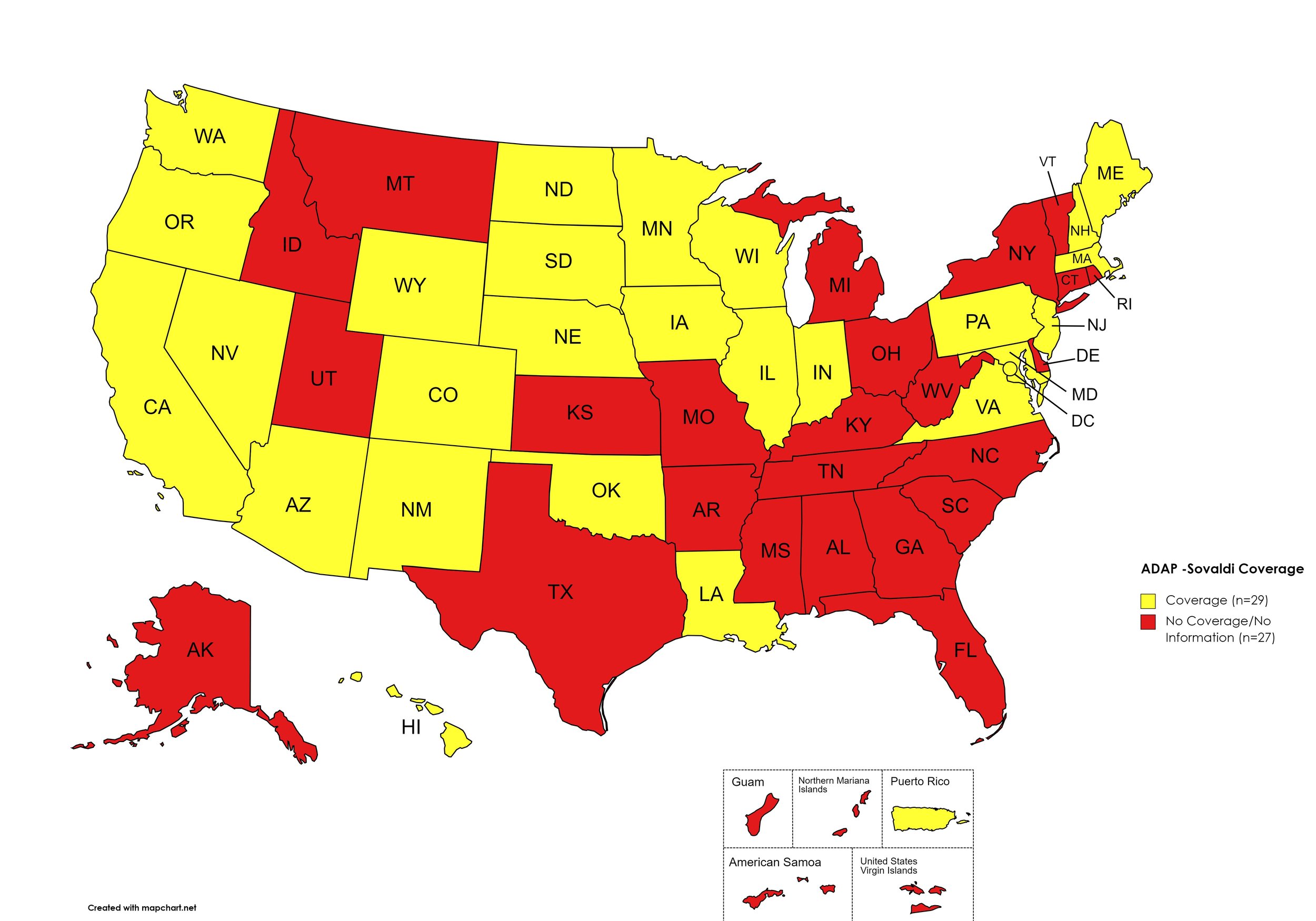
Sovaldi
States with Sovaldi Coverage: AZ, CA, CO, HI, IL, IN, IA, LA, ME, MD, MA, MN, NE, NV, NH, NJ, NM, ND, OK, OR, PA, SD, VA, WA, WI, WY, D.C.
States without Sovaldi Coverage: AL, AK, AR, CT, DE, FL, GA, ID, KS, KY, MI, MS, MO, MT, NY, NC, OH, RI, SC, TN, TX, UT, VT, WV
Territories with Sovaldi Coverage: P.R.
Figure 2. October 2023 ADAP Coverage - Sovaldi
Map Key: Yellow = Sovaldi Coverage; Red = No Sovaldi Coverage/No Information regarding Sovaldi Coverage
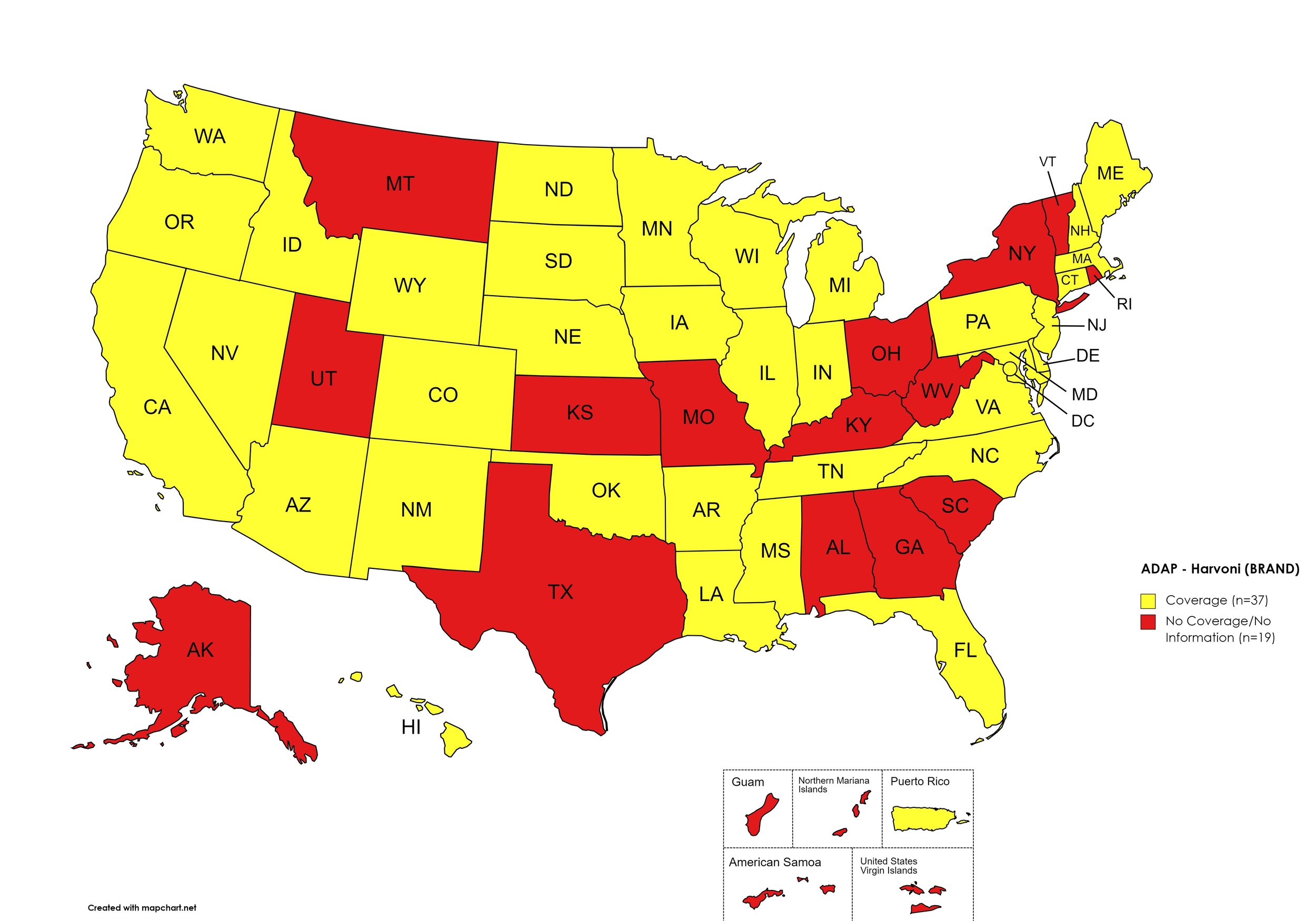
Harvoni
States with Harvoni Coverage: AZ, AR, CA, CO, CT, DE, FL, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, NE, NV, NH, NJ, NM, NC, ND, OK, OR, PA, SD, TN, VA, WA, WI, WY, D.C.
States without Harvoni Coverage: AL, AK, GA, KS, KY, MO, MT, NY, OH, RI, SC, TX, UT, VT, WV
Territories with Harvoni Coverage: P.R.
Figure 3. October 2023 ADAP Coverage - Harvoni
Map Key: Yellow = Harvoni Coverage; Red = No Harvoni Coverage/No Information regarding Harvoni Coverage
Zepatier
States with Zepatier Coverage: AL, AZ, AR, CA, CO, FL, GA, HI, IL, IA, LA, ME, MD, MA, MI, MN, MS, NE, NV, NH, NJ, NM, NY, NC, ND, OR, PA, SD, VA, WA, WV, WI, WY, D.C.
States without Zepatier Coverage: AK, CT, DE, ID, IN, KS, KY, MO, MT, OH, OK, RI, SC, TN, TX, UT, VT
Territories with Zepatier Coverage: P.R.
Figure 4. October 2023 ADAP Coverage - Zepatier
Map Key: Yellow = Zepatier Coverage; Red = No Zepatier Coverage/No Information regarding Zepatier Coverage
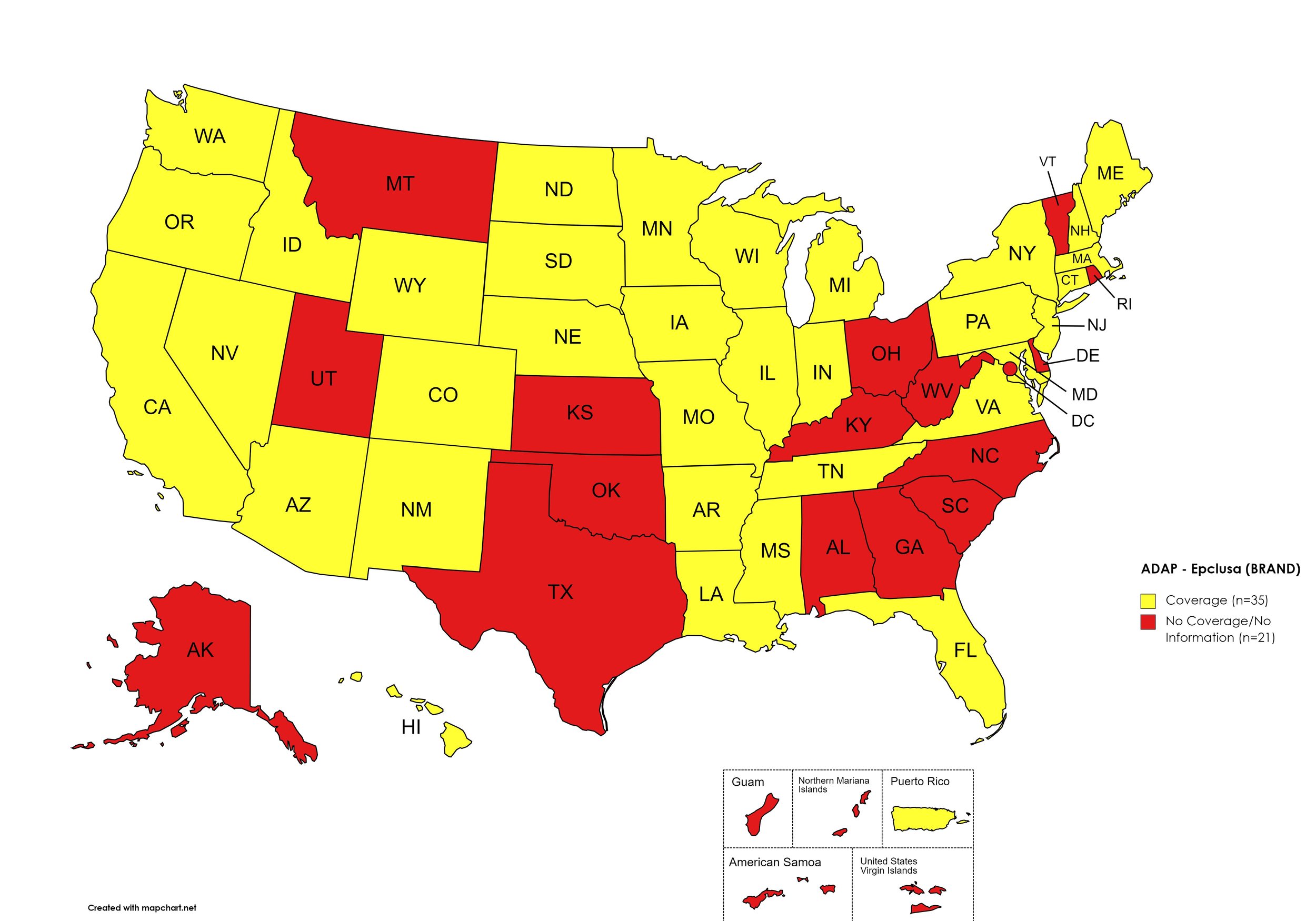
Epclusa
States with Epclusa Coverage: AZ, AR, CA, CO, CT, FL, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, MO, NE, NY, NV, NH, NJ, NM, ND, OR, PA, SD, TN, VA, WA, WI, WY
States without Epclusa Coverage: AL, AK, DE, GA, KS, KY, MT, NC, OH, OK, RI, SC, TX, UT, VT, WV, D.C.
Territories with Epclusa Coverage: P.R.
Figure 5. October 2023 ADAP Coverage - Epclusa
Map Key: Yellow = Epclusa Coverage; Red = No Epclusa Coverage/No Information regarding Epclusa Coverage
Vosevi
States with Vosevi Coverage: CA, CT, FL, HI, ID, IL, IN, IA, LA, MD, MA, MN, NE, NV, NH, NJ, NM, ND, OR, SD, TN, WA, WY
States without Vosevi Coverage: AL, AK, AZ, AR, CO, DE, GA, KS, KY, ME, MI, MS, MO, MT, NY, NC, OH, OK, PA, RI, SC, TX, UT, VT, VA, WV, WI, D.C.
Territories with Vosevi Coverage: P.R.
Figure 6. October 2023 ADAP Coverage - Vosevi
Map Key: Yellow = Vosevi Coverage; Red = No Vosevi Coverage/No Information regarding Vosevi Coverage
Mavyret
States with Mavyret Coverage: AL, AZ, AR, CA, CO, CT, FL, GA, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OR, PA, SD, TN, VA, WA, WV, WI, WY, D.C.
States without Mavyret Coverage: AK, DE, KS, KY, OH, OK, RI, SC, TX, UT, VT
Territories with Mavyret Coverage: P.R.
Figure 7. October 2023 ADAP Coverage - Mavyret
Map Key: Yellow = Mavyret Coverage; Red = No Mavyret Coverage/No Information regarding Mavyret Coverage
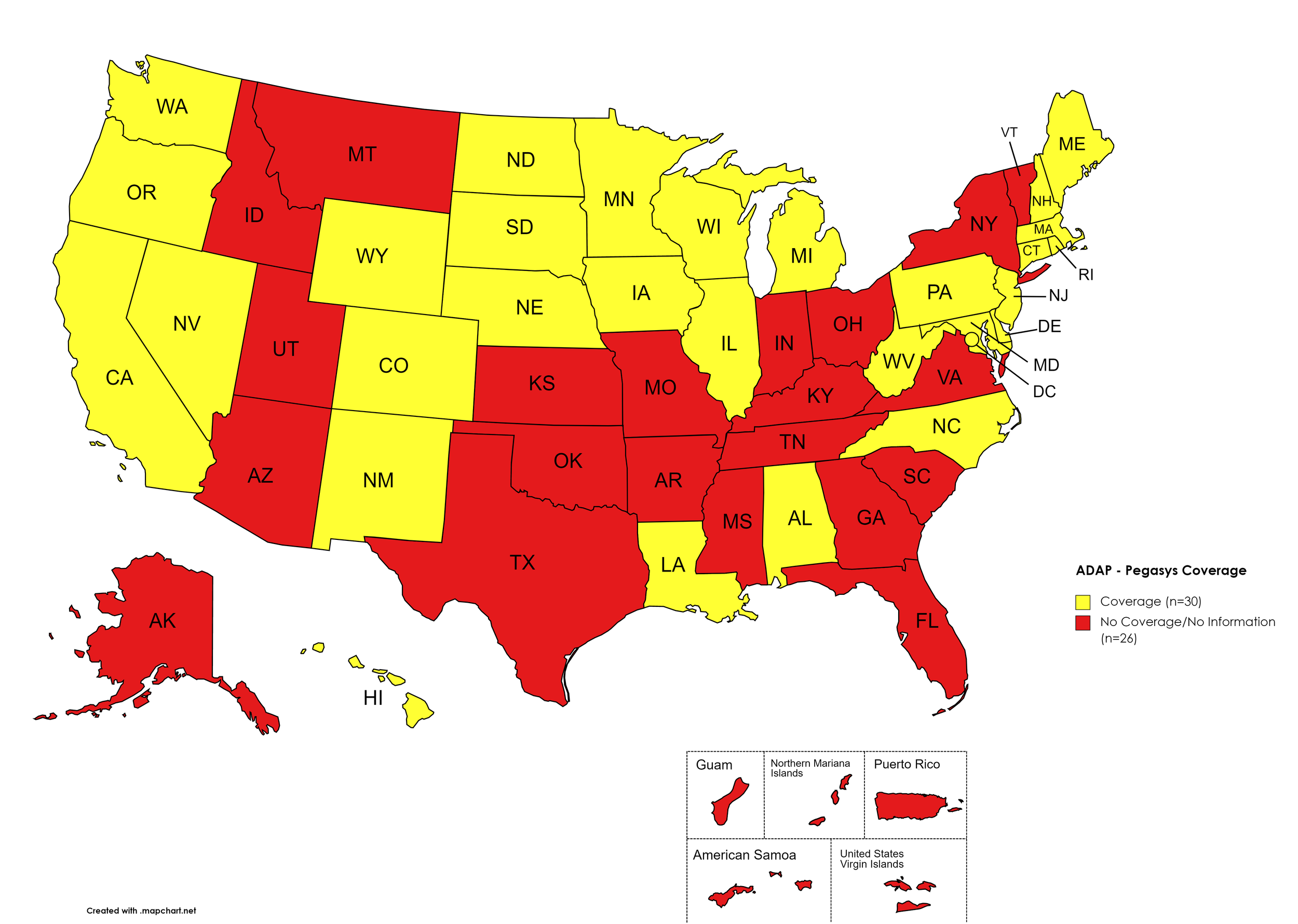
Pegasys
States with Pegasys Coverage: AL, CA, CO, CT, DE, HI, IL, IA, LA, ME, MD, MA, MI, MN, NE, NV, NH, NJ, NM, NC, ND, OR, PA, RI, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Pegasys Coverage: AK, AZ, AR, FL, GA, ID, IN, KS, KY, MS, MO, MT, NY, OH, OK, SC, TN, TX, UT, VT, VA
Territories with Pegasys Coverage: None/Unknown
Figure 8. October 2023 ADAP Coverage - Pegasys
Map Key: Yellow = Pegasys Coverage; Red = No Pegasys Coverage/No Information regarding Pegasys Coverage
Harvoni (generic)
States with Harvoni (generic) Coverage: AZ, AR, CA, CO, CT, FL, GA, IL, IA, ME, MD, MA, MN, MS, NE, NV, NH, NJ, NM, NC, ND, OK, OR, PA, SD, TN, WA, WI, WY, D.C.
States without Harvoni (generic)Coverage: AL, AK, DE, HI, ID, IN, KS, KY, LA, MI, MO, MT, NY, OH, RI, SC, TX, UT, VT, VA, WV
Territories with Harvoni (generic) Coverage: P.R.
Figure 9. October 2023 ADAP Coverage - Harvoni (Generic)
Map Key: Yellow = Harvoni (Generic) Coverage; Red = No Harvoni (Generic) Coverage/No Information regarding Harvoni (Generic) Coverage
Epclusa (generic)
States with Epclusa (generic) Coverage: AZ, AR, CA, CO, CT, FL, GA, IL, IN, IA, ME, MD, MA, MN, MS, MO, NE, NV, NH, NJ, NM, ND, OR, PA, SD, TN, WA, WI, WY, D.C.
States without Epclusa (generic) Coverage: AL, AK, DE, HI, ID, KS, KY, LA, MI, MT, NY, NC, OH, OK, RI, SC, TX, UT, VT, VA, WV
Territories with Epclusa (generic) Coverage: P.R.
Figure 10. October 2023 ADAP Coverage - Epclusa (generic)
Map Key: Yellow = Epclusa (generic) Coverage; Red = No Epclusa (generic) Coverage/No Information regarding Epclusa (generic) Coverage
October 2023 Notes:
States with Open Formularies: IL, IA, MA, MN, NE, NH, NJ, NM, ND, OH, OR, WA, WY
N.B. – Although Ohio is listed by NASTAD as having an open formulary, both NASTAD’s ADAP Formulary Database and Ohio’s ADAP website indicates that the state does not offer any treatment for HCV.
N.B. – Although North Dakota has adopted an open formulary, they provide only co-pay and deductible assistance for HCV medications.
N.B. – Wyoming's ADAP Open Formulary document, the following disclaimer related to HCV is made: Hepatitis C treatment medications (i.e. Harvoni, Sovaldi, Ribavirin, Zepatier, Epclusa) must be prior authorized. To be eligible, clients must have applied for prior authorization from their insurance plan and the WY ADAP Hepatitis C Treatment checklist must be completed and signed by the provider and client.
Colorado offers five coverage options – Standard ADAP, HIV Medical Assistance Program (HMAP), Bridging the Gap Colorado (BTGC), HIV Insurance Assistance Program (HIAP), and Supplemental Wrap Around Program (SWAP). ‘Yes’ indications in Figure 1. for Colorado denote that at least one of these programs offers coverage for each respective drug. The Standard ADAP Formulary covers medications only if funds are available to do so.
Louisiana’s ADAP (Louisiana Health Access Program – LA HAP) offers two coverage options – Uninsured (Louisiana Drug Assistance Program – L-DAP) and Insured (Health Insurance Program – HIP). HIP pays for the cost of treatment only if the client’s primary insurance covers the drug under its formulary.
On August 11th, the Georgia Department of Public Health issued a notice to Ryan White Part B District Coordinators, reading, in part, “Effective 8/14/2023, care providers will have the ability to order Hepatitis C medications for their eligible ADAP patients without the need for Prior Approval.” Initially covered medications are limited to ribavirn, Zepatier, Mavyret, and generics for Epclusa and Harvoni.
Hawaii’s ADAP notes the following: “Treatment slots for HCV direct-acting antivirals may be limited. Prescriber or pharmacy must call HDAP for slot.”
Texas ADAP maintains no HCV coverage, despite a brief period of covering DAAs in 2022.
California’s ADAP pharmacy benefit manager has recently removed coverage of ribavirin products for the treatment of HCV.
3. MEDICAID PROGRAMS & HCV THERAPIES
All 50 states and the District of Columbia continue to offer some form of HCV coverage. All 50 states and the District of Columbia have expanded their Preferred Drug Lists to include at least one HCV Direct Acting Agent (DAA).
October 2023 Updates:
Basic Coverage
States with Basic HCV Medications Coverage: AZ, AK, AR, CA, CO, CT, DE, FL, GA, HI, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OR, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Basic HCV Medications Coverage: AL, ID, KS, MO, OK, SC, VA, WY
Figure 11. October 2023 Medicaid Coverage - Basic HCV Medications
Map Key: Blue = Basic HCV Medication Coverage; Yellow = No Basic HCV Medication Coverage/No Information regarding Basic HCV Medication Coverage
Sovaldi
States with Sovaldi Coverage: AR, CA, CO, DE, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WI, D.C.
States without Sovaldi Coverage: AL, AK, AZ, CT, FL, IA, NH, NM, NV, OK, OR, SC, VA, WV, WY.
Figure 12. October 2023 Medicaid Coverage - Sovaldi
Map Key: Blue = Sovaldi Coverage; Yellow = No Sovaldi Coverage/No Information regarding Sovaldi Coverage
Harvoni
States with Harvoni Coverage: AL, AR, CA, CO, DE, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NH, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Harvoni Coverage: AK, AZ, CT, FL, IA, NV, NM, OK, OR, SC, VA, WY.
Figure 13. October 2023 Medicaid Coverage - Harvoni
Map Key: Blue = Harvoni Coverage; Yellow = No Harvoni Coverage/No Information regarding Harvoni Coverage
Zepatier
States with Zepatier Coverage: AL, AR, CA, CO, DE, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WI, D.C.
States without Zepatier Coverage: AK, AZ, CT, FL, IA, NV, NH, NM, OK, OR, SC, VA, WV, WY.
Figure 14. October 2023 Medicaid Coverage - Zepatier
Map Key: Blue = Zepatier Coverage; Yellow = No Zepatier Coverage/No Information regarding Zepatier Coverage
Epclusa
States with Epclusa Coverage: AL, AR, CA, CO, DE, GA, HI, IL, IN, KS, KY, LA, MA, ME, MI, MN, MS, MO, MT, NH, NJ, NM, NY, NC, ND, OH, OR, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Epclusa Coverage: AK, AZ, CT, FL, ID, IA, MD, NE, NV, OK, SC, VA, WY.
Figure 15. October 2023 Medicaid Coverage - Epclusa
Map Key: Blue = Epclusa Coverage; Yellow = No Epclusa Coverage/No Information regarding Epclusa Coverage
Vosevi
States with Vosevi Coverage: AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NH, NJ, NY, NC, ND, OH, PA, RI, SC, SD, TN, TX, UT, VT, WA, WI, D.C.
States without Vosevi Coverage: AL, AK, AZ, NV, NM, OK, OR, VA, WV, WY.
Figure 16. October 2023 Medicaid Coverage - Vosevi
Map Key: Blue = Vosevi Coverage; Yellow = No Vosevi Coverage/No Information regarding Vosevi Coverage
Mavyret
States with Mavyret Coverage: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
Figure 17. October 2023 Medicaid Coverage - Mavyret
Map Key: Blue = Mavyret Coverage; Yellow = No Mavyret Coverage/No Information regarding Mavyret Coverage
Pegasys
States with Pegasys Coverage: AK, AZ, CA, CT DE, FL, GA, HI, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MS, MT, NE, NV, NH, NJ, NM, NY, NC, OH, OR, PA, RI, SD, TN, TX, VT, WA, WV, WI, D.C.
States without Pegasys Coverage: AL, AR, CO, ID, KS, MO, ND, OK, SC, UT, VA, WY
Figure 18. October 2023 Medicaid Coverage - Pegasys
Map Key: Blue = Pegasys Coverage; Yellow = No Pegasys Coverage/No Information regarding Pegasys Coverage
Harvoni (generic)
States with Harvoni (generic) Coverage: AL, AR, CA, CO, DE, GA, HI, ID, IL, IN, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Harvoni (generic) Coverage: AK, AZ, CT, FL, IA, KS, NM, OK, OR, SC, VA, WY
Figure 19. October 2023 Medicaid Coverage - Harvoni (generic)
Map Key: Blue = Harvoni (generic) Coverage; Yellow = No Harvoni (generic) Coverage/No Information regarding Harvoni (generic) Coverage
Epclusa (generic)
States with Epclusa (generic) Coverage: AK, AL, AZ, AR, CA, CO, CT, DE, FL, GA, HI, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OR, PA, RI, SC, SD, TN, TX, VT, VA, WA, WV, WI, WY, D.C.
States without Epclusa (generic) Coverage: ID, OK, UT
Figure 20. October 2023 Medicaid Coverage - Epclusa (generic)
Map Key: Blue = Epclusa (generic) Coverage; Yellow = No Epclusa (generic) Coverage/No Information regarding Epclusa (generic) Coverage
October 2023 Notes:
The follow states’ Medicaid programs offer multiple coverage plans for their respective Medicaid clients. The plan highlighted in bold typeface represents the most comprehensive plan with the most drugs covered in the respective state:
Hawaii – (1.) Advantage Plus; (2.) QUEST Integration
New Jersey – (1.) Aetna; (2.) AmeriGroup NJ; (3.) Horizon NJ Health; (4.) UnitedHealthcare of New Jersey; (5.) WellCare
New Mexico – (1.) BlueCross BlueShield of New Mexico; (2.) Presbyterian Centennial Care; (3) Western Sky Community Care
Kentucky has a Unified Medicaid Formulary
Louisiana has a Unified Medicaid Formulary
Ohio – Ohio has a Unified Medicaid Formulary that applies to all MCOs
Nevada’s Medicaid plan removed coverage for Sovaldi, Harvoni (brand and generic), Zepatier, Epclusa (brand only), and Vosevi.
Utah’s Medicaid plan removed coverage for Epclusa (generic)
No data is has been made available by the Medicaid programs in the U.S. Territories.
*Medicaid coverage excludes patients from most drug manufacturer patient assistance programs (PAPs)
4. VETERANS PROGRAMS & HCV THERAPIES
The Veteran's Administration (VA) currently offers coverage for all HCV drugs. This is according to the most recent VA National Formulary, dated May 2021 (U.S. Dept. of V.A., 2021a). The VA Treatment Considerations and Choice of Regimen for HCV-Mono-Infected and HIV/HCV Co-Infected Patients, dated March 2021 (U.S. Dept. of V.A., 2021b) lists the following therapies as preferred treatments:
Abbreviations:
- CTP – Child-Turcotte-Pugh (score used to assess severity of cirrhosis)
- IU/mL – International Units Per Milliliter
- PEG-IFN/IFN – Peginterferon/Interferon
- RAS – Resistance-associated substitutions
Genotype 1:
Treatment-naïve without or with cirrhosis (CTP A):
Pangenotypic regimens
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens:
Zepatier: 1 tablet orally daily for 12 weeks if GT1a without baseline NS5A RAS or GT1b
Harvoni: 1 tablet orally daily
If HCV-noninfected, non-cirrhotic, and HCV RNA baseline <6 million IU/mL: 8 weeks
If cirrhotic, baseline HCV RNA ≥6 million IU/mL, HIV/HCV-co-infected, or African American: 12 weeks
Consider adding ribavirin in CTP A patients
Treatment-naïve with decompensated cirrhosis (CTP B or C):
Harvoni: 1 tablet orally daily + ribavirin (600 mg/day and increase by 200 mg/day every 2 weeks only as tolerated) for 12 weeks
Epclusa: 1 tablet orally daily + ribavirin (1000 mg/day - <75kg – or 1,200 mg daily - ≥75kg – orally daily in 2 divided doses with food) for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb).
Treatment-experienced (NS5A- and SOF-naïve [e.g., failed PEG-IFN/RBV ± NS3/4A PI]) without or with cirrhosis (CTP A)
Pangenotypic regimens:
Mavyret: 3 tablets orally daily with food
If PEG-IFN/RBV-experienced: 8 weeks if non-cirrhotic or 12 weeks if cirrhotic
If NS3/4A PI + PEG-IFN/RBV-experienced: 12 weeks
Vosevi: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens
Zepatier: 1 tablet orally daily for 12 weeks if GT1b, or if failed only PEG-IFN/RBV and GT1a without baseline NS5A RAS
Harvoni: 1 tablet orally daily for 12 weeks
Treatment-experienced (NS5A-naïve and SOF-experienced) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food
If PEG-IFN/RBV + Sovaldi-experienced: 8 weeks if non-cirrhotic or 12 weeks if cirrhotic
If Olysio + Sovaldi-experienced: 12 weeks
Epclusa: 1 tablet orally daily for 12 weeks if GT1b
Vosevi: 1 tablet orally daily with food for 12 weeks if GT1a
Treatment-experienced (prior NS5A-containing regimen) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 16 weeks if failed only an NS5A inhibitor without NS3/4A PI (e.g., Harvoni)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-experienced with decompensated cirrhosis (CTP B or C)
Epclusa: 1 tablet orally daily + RBV; start at lower RBV doses as clinically indicated (e.g., baseline Hgb);
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks; NOT FDA approved for 24 weeks
Genotype 2:
Treatment-naïve or treatment-experienced (PEG-IFN/IFN ± RBV or Sovaldi + RBV ± PEG-IFN) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks; 12 weeks if CTP A and treatment-experienced or in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-naïve or treatment-experienced patients with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks
Genotype 3:
Treatment-naïve without cirrhosis or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks if cirrhotic or in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
If CTP A, test for NS5A RAS
Add ribavirin if Y93H RAS present
Treatment-experienced (PEG-IFN ± RBV or Sovaldi + RBV ± PEG-IFN) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 16 weeks
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
If CTP A, consider adding ribavirin (no supporting data)
Treatment-naïve or treatment-experienced with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks
Genotype 4:
Treatment-naïve without or with cirrhosis (CTP A)
Pangenotypic regimens
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens
Zepatier: 1 tablet orally daily for 12 weeks
Harvoni: 1 tablet orally daily for 12 weeks
Treatment-naïve with decompensated cirrhosis (CTP B or C)
Pangenotypic regimen
Epclusa: 1 tablet orally daily + RBV for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
Non-pangenotypic regimen:
Harvoni: 1 tablet orally daily + ribavirin (600 mg/day and increase by 200 mg/day every 2 weeks only as tolerated) for 12 weeks
Treatment-experienced (Sovaldi-experienced and NS5A-naïve) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks if NS3/4A PI-naïve without cirrhosis, and 12 weeks if NS3/4A PI-experienced or CTP A
Epclusa: 1 tablet orally daily + ribavirin for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-experienced with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks; NOT FDA approved for 24 weeks
5. PATIENT ASSISTANCE PROGRAMS
The drug manufacturers and various national nonprofit organizations offer a variation of patient assistance programs (PAPs) to assist patients in accessing treatments. They include:
Support Path (Gilead Sciences):
Financial Assistance
Provides Co-Pay Coupons for Sovaldi, Harvoni, Harvoni (Generic), Epclusa, Epclusa (Generic), and Vosevi
Co-Pay Coupons cover out-of-pocket costs up to 25% of the catalog price of a 12-week regimen (3 bottles/packages) of Sovaldi, Harvoni, Harvoni (Generic), Epclusa, Epclusa (Generic), or Vosevi
Excludes patients enrolled in Medicare Part D or Medicaid
Insurance Support
Researches and verifies patient’s benefits, and gives information they need about coverage options and policies
Explain Prior Authorization process and works with HCV Specialist’s office so they can submit PA forms to a patient’s insurance company
May be able to provide assistance with appeals process
Website: http://www.mysupportpath.com/
AbbVie Mavyret Co-Pay Savings Card:
Financial Assistance
Patient may be eligible to pay as little as $5
Excludes patients enrolled in Medicare Part D, Medicare Advantage, Medigap, Medicaid, TRICARE, Department of Defense, or Veterans Affairs programs)
NeedyMeds:
NeedyMeds Drug Discount Card
Designed to lower cost of prescription medications by up to 80% at participating pharmacies
Price finder tool for the drug discount card
No eligibility requirements
CANNOT be used in combination with government healthcare programs, but CAN be used IN PLACE of program
CANNOT be combined with other offers
Website: http://ow.ly/fEJo309cJ7Z
The Assistance Fund:
Status: WAITLISTED
Requires provider referral
Copay assistance
Eligibility Criteria:
US citizen or permanent resident
Diagnosed with the disease for which you are applying
Prescribed an FDA-approved treatment for the disease
Have prescription coverage for the prescribed treatment
Meet financial eligibility criteria based upon household income and size
Patient Advocate Foundation Co-Pay Relief:
Status: CLOSED
Maximum award of $15,000
Eligibility Requirements:
Patient must be insured, and insurance must cover prescribed medication
Confirmed HCV diagnosis
Reside and receive treatment in the U.S.
Income falls below 400% of FPL with consideration of the Cost of Living Index (COLI) and the number in the household
Patient Access Network (PAN) Foundation:
Status: OPEN
Co-Pay Assistance with a maximum award of $5,600
Patients may apply for additional assistance during their eligibility period, subject to availability of funding
Eligibility Requirements:
Must be getting treatment for HCV
Have insurance that covers prescribed HCV medication
Medication must be listed on PAN’s list of covered medications: https://www.panfoundation.org/index.php/en/patients/medications-covered
Income falls below 500% of FPL
Residing and receiving treatment in the U.S. (citizenship NOT required)
Website: https://www.panfoundation.org/index.php/en/patients/assistance-programs/hepatitis-c
HealthWell Foundation:
Status: OPEN
Co-Pay Assistance with a maximum award of $30,000
Minimum Co-Pay Reimbursement Amount: None
Minimum Premium Reimbursement Amount: None
Eligibility Requirements:
Must be being treated for HCV
Have insurance that covers HCV prescribed medication
Income falls below 500% of FPL
Receiving treatment in the U.S.
Website: https://www.healthwellfoundation.org/fund/hepatitis-c/
6. HARM REDUCTION PROGRAMS
Harm Reduction, as it relates to opioid abuse and HCV, are measures designed to serve as preventive or monitoring efforts in combating opioid prescription drug and heroin abuse, and as an effect, helping to prevent the spread of HCV and HIV. The Co-Infection Watch covers the following measures: Syringe Exchange, Expanded Naloxone Access, Good Samaritan Laws, Mandatory PDMP Reporting, Doctor Shopping Laws, Physical Exam Requirements, ID Requirements for Purchase, Required or Recommended Prescriber Education, and Lock-In Programs (Editor’s Note: Program descriptions provided herein).
October 2023 Updates:
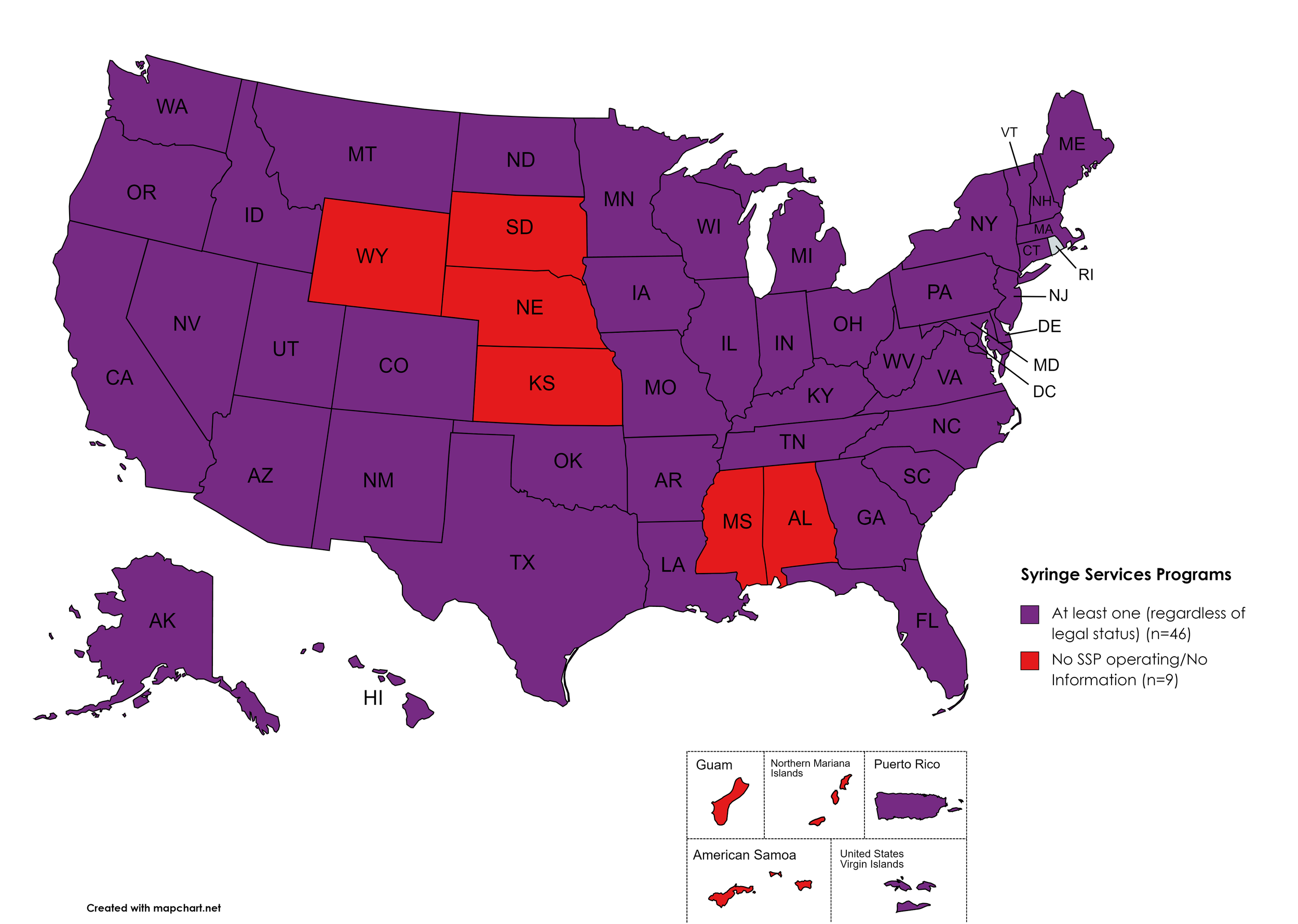
Syringe Exchange
Syringe Services Programs (SSPs) exist to provide injection drug users (or those whose prescriptions require injection) with clean syringes and/or in exchange for used ones. (N.b. – states listed as "at least one SSP…” indicate only that a Syringe Services Program (SSP) exists within the state, regardless of the legality of SSPs under state law).
States with Syringe Exchange: AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MO, MT, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, D.C.
States without Syringe Exchange: AL, KS, MS, NE, SD, WY
Territories with Syringe Exchange: Puerto Rico, U.S. Virgin Islands
Figure 21. October 2023 Syringe Exchange Coverage
Map Key: Purple = Syringe Exchange(s); Red = No Syringe Exchange(s); Grey = No Information
Expanded Naloxone
Naloxone is a drug used to counteract the effects of opioid overdoses. Expanded Access refers to one of more of the following conditions: Naloxone purchase without a prescription; availability to schools, hospitals, and emergency response units for use in the event of an overdose.
States with Expanded Naloxone: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Expanded Naloxone: None
Territories with Expanded Naloxone: Unknown
Figure 22. October 2023 Expanded Naloxone Coverage
Map Key: Purple = Expanded Naloxone; Red = Restricted Naloxone; Gray = No Information
Good Samaritan Laws
Good Samaritan Laws are laws that are designed to protect emergency services personnel, public or private employees, and/or citizens from being held legally liable for any negative healthcare outcomes as a result of providing "reasonable measures" of emergent care.
States with Samaritan Laws: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Samaritan Laws: None
Territories with Samaritan Laws: Unknown
Figure 23. October 2023 Good Samaritan Laws Coverage
Map Key: Purple = Good Samaritan Laws; Red = No Good Samaritan Laws; Gray: No Information
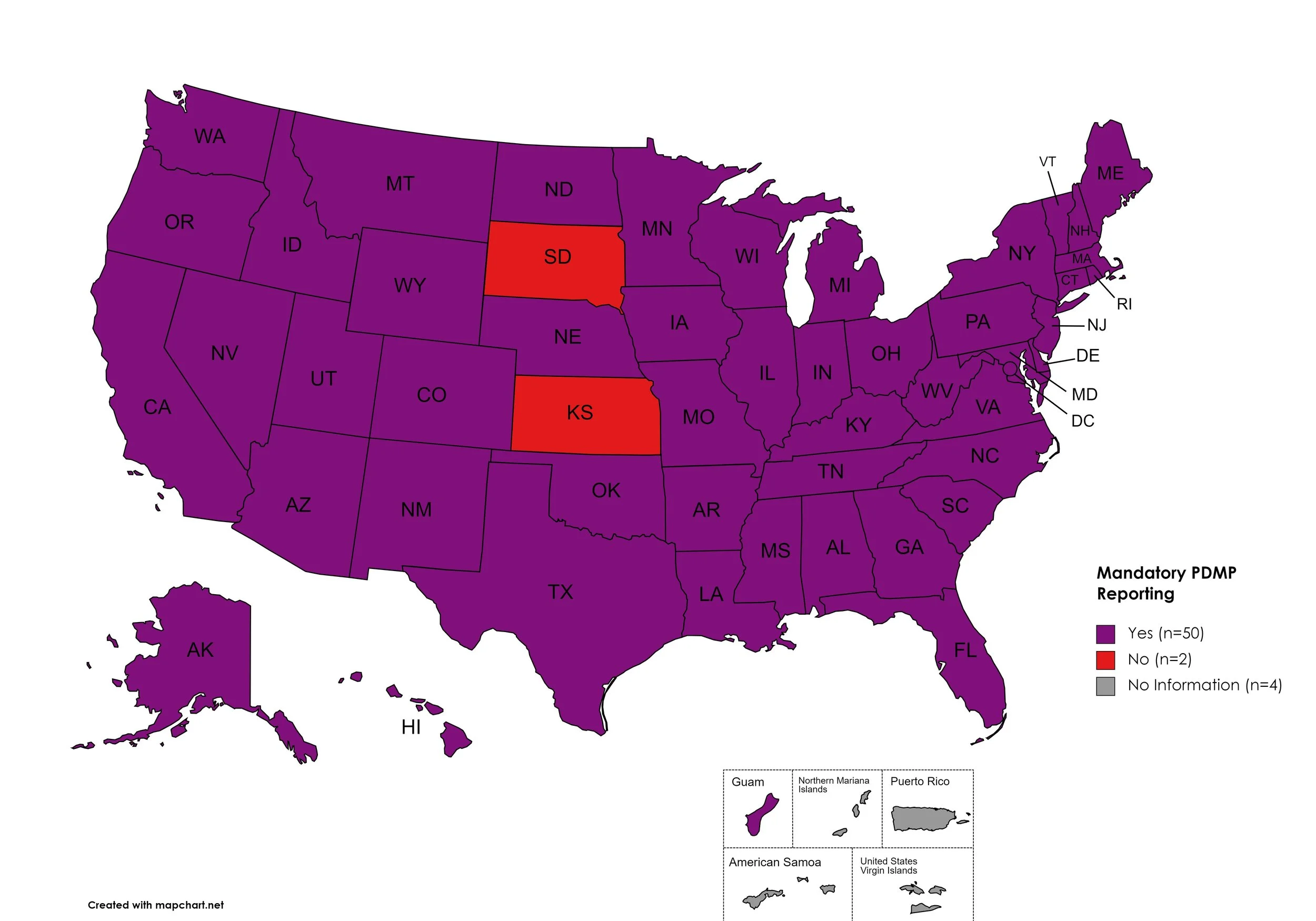
Mandatory PDMP Reporting
Prescription Drug Monitoring Programs (PDMPs) are programs established by state and/or federal law that requires prescribing physicians and/or the fulfilling pharmacies to register with a state agency. While every state has established required enrollment in a PDMP for prescribers, not all states mandate dispensing entities to enroll. Similarly, prescription reporting to a state agency may include one or more of the following data points: Patient Names; Specific Drug(s) Prescribed; Prescription Dosage; Date; Time; Form of State-Issued ID.
States with Mandatory PDMP Reporting: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MO, MS, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Mandatory PDMP Reporting: SD, KS
Territories with Mandatory PDMP Reporting: Guam
Figure 24. October 2023 Mandatory Prescription Drug Monitoring Program Coverage
Map Key: Purple = Mandatory PDMP; Red = No Mandatory PDMP; Gray = No Information
Doctor Shopping Laws
Doctor Shopping Laws are those laws designed to prevent patients from seeking one or more of the same prescription from multiple doctors through the use of subterfuge, falsifying identity, or any other deceptive means. While federal law prohibits Doctor Shopping, most states also include provisions that prohibit patients from seeking a new prescription if another physician has denied a similar prescription within a certain period of time.
States with Doctor Shopping Laws: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Doctor Shopping Laws: None
Territories with Doctor Shopping Laws: None
Figure 25. October 2023 Doctor Shopping Laws Coverage
Map Key: Purple = Doctor Shopping Laws; Red = No Doctor Shopping Laws; Grey = No Information
Physical Exam Required
Physical Exam Requirements are those that mandate that the prescribing physician perform a physical examination on a patient before providing a prescription for a controlled substance to determine if the prescription is medically necessary. Many states have expanded their definitions of “physical exam” to include a physical exam via telehealth when such an exam is similarly significant and sufficient as being seen in-person.
States with Physical Exam Required: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, MD, MA, ME, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, PA, RI, SC, SD, TN, TX, UT, VA, VT, WA, WV, WI, WY, D.C.
States without Physical Exam Required: SD
Territories with Physical Exam Required: None
Figure 26. October 2023 Physical Exam Required Coverage
Map Key: Purple = Physical Exam Required; Red: No Physical Exam Required; Grey = No Information
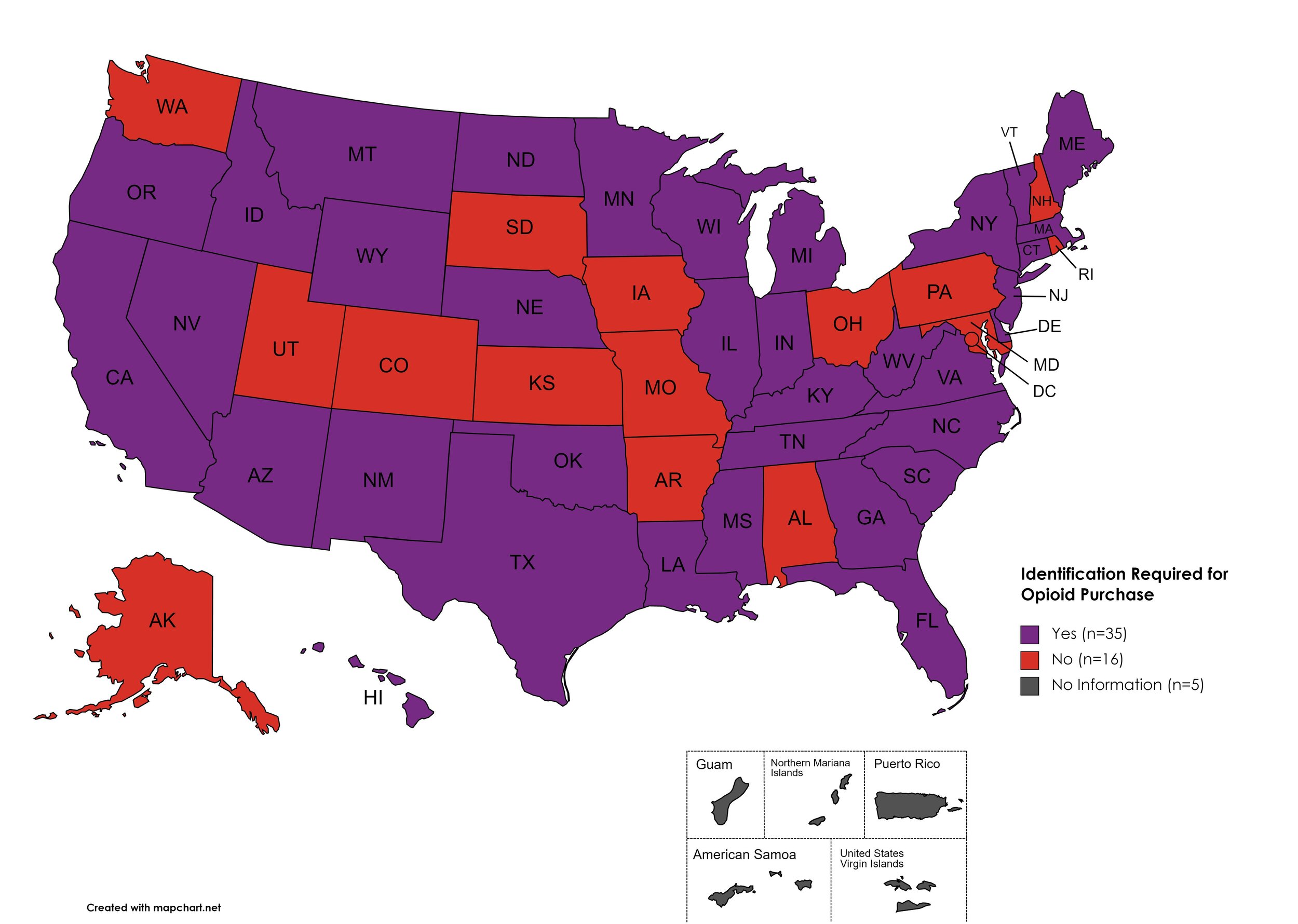
I.D. Required for Purchase of Opioid Prescription
Federal law requires anyone purchase a controlled substance to provide a state-issued identification (“I.D.”) in order to fill the prescription. Mandatory ID requirements go further and require that this information be recorded and stored in an effort to prevent the same patient from obtaining multiple or repeated prescriptions in a given period of time.
States with I.D. Required: AZ, CA, CT, DE, FL, GA, HI, ID, IL, IN, KY, LA, ME, MA, MI, MS, MN, MT, NE, NV, NJ, NM, NY, NC, ND, OK, OR, SC, TN, TX, VT, VA, WV, WI, WY
States without I.D. Required: AL, AK, AR, CO, IA, KS, MD, MO, NH, OH, PA, RI, SD, UT, WA, D.C.
Territories with I.D. Required: Unknown
Figure 27. October 2023 I.D. Required Coverage
Map Key: Purple = I.D. Required; Red = No I.D. Required; Gray = No Information
Prescriber Education Required
States that require/do not require that prescribing physicians undergo special training in addition to or as part of their initial education to become prescribers related to safer controlled substance and/or pain management prescribing and utilization practices.
States with Prescriber Education Required: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MO, MN, MS, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Prescriber Education Required: MT, SD
Territories with Prescriber Education Required: Unknown
Figure 28. October 2023 Prescriber Education Required Coverage
Map Key: Purple = Prescriber Ed Required; Red = No Prescriber Ed Required; Gray = No Information
Medicaid Lock-In Program
Lock-In Programs are laws requiring that patients either receive prescriptions from only one physician and/or fill prescriptions from only one pharmacy.
States with Medicaid Lock-In Program: AL, AK, AZ, AR, CA, CO, CT, DE, GA, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Medicaid Lock-In Program: FL, HI, SD
Territories with Medicaid Lock-In Program: Unknown
Figure 29. October 2023 Medicaid Lock-In Coverage
Map Key: Purple = Medicaid Lock-In; Red = No Medicaid Lock-In; Gray = No Information
October 2023 Notes:
Metric definition for Mandatory PDMP Reporting section has been updated for clarity, the new map reflects the following:
Mandated prescriber and/or dispenser reporting only and do not reflect mandatory enrollment of prescribers or dispensers.
Most states do not require enrollment or of or reporting from both prescribers and dispensers; rather, most states with requirements require one or the other.
Many states have specific exceptions for reporting.
This adjustment clarifies that SD and KS are the only two states without required PDMP reporting by a prescriber or dispenser.
This adjustment clarifies that MT does require PDMP reporting by a prescriber or dispenser.
Metric definition for Physician Education has been updated to exclude “recommended” and only reflect those states which have laws or licensing board requirements of initial and/or continuing education for prescribers with regard to pain management and/or the prescription of controlled substances.
Some states have general requirements regarding “controlled substances”, some states are explicit with regard to category of controlled substance or type of controlled substance (ie. “opioids”).
This adjustment clarifies that MT and SD are the only states that do not require opioid specific and/or pain management specific and/or controlled substances prescribing education by law or licensing institution in either core or continuing education for providers.
This adjustment clarifies that KS, MO, and ND do require opioid specific and/or pain management specific and/or controlled substances prescribing education by law or licensing institution in either core or continuing education for providers.
Metric definition for Physical Exam has been updated for clarity regarding telehealth.
Many states have integrated statutory language requiring an exam in order to prescribe medication or otherwise establish an authentic patient-provider relationship to include telemedicine technologies. These technologies must be similarly sufficient to meet the standards of care and facilitate a similarly situated experience as to an in-person exam.
This adjustment clarifies that SD is now the only state which does not require a physical exam in-person or via sufficiently similar telehealth methods.
This adjustment means KS, MT, OR, and WI laws capture an exam requirement in order for a provider to establish an authentic patient-provider relationship and prescribe medications, including controlled substances.
CANN is no longer able to independently verify the existence of an SSP in Kansas. KS state laws prohibit SSPs and syringes are included in the state’s drug paraphernalia law.
The DEA’s COVID-19-based waiver of in-person exams is expected to expire in November of 2023. A final rule on this change has not yet been published as of the time of this report. The DEA’s proposed rule was met with sufficient enough advocate, patient, and provider objection that it will not be taken up and a new proposed rule is being drafted as of the time of this writing. News: The DEA and HHS have again extended the full slate of tele-prescribing flexibilities allowed during the COVID-19 Public Health Emergency through December 31, 2024.
CANN is in the process of reviewing monitored metrics for “Harm Reduction” and will be providing an update in the coming Watch.
7. COVID-19 IMPACT ON HIV & HCV
The Community Access National Network’s blog began 2021 by assessing COVID-19’s impact on HIV, HCV, and Substance-Use Disorder. We will continue to monitor developments in light of the ongoing COVID-19 pandemic and its impacts on public health until January 2024. Beginning in January 2024, issues related to public health policy stemming from COVID-19 impacts will be included in the “Latest News” section of the Watch.
Additional Resources and Relevant Issues:
DEA Extends Telehealth Prescribing Flexibilities Through 2024 - As the Drug Enforcement Agency (DEA) and U.S. Department of Health and Human Services (HHS) struggle to identify an approach telehealth prescribing of controlled substances that won’t leave patients and providers screaming, because the agencies jointly announced another extension of flexibilities provided during the COVID-19 pandemic. The extension allows for the “full set of telemedicine flexibilities”, which were earlier slated to end in early November 2023. The extension is now slated to last until December 31, 2024. The longer these flexibilities go on, the harder it will be for the DEA to roll back these flexibilities, rather than integrating allowable telehealth prescribing as a standard practice (an option which appears to be on the table), as advocates for greater access argue such changes appear to be “arbitrary and capricious”, working against access gains made during the public health emergency. Some of the controlled substances in question include opioids, medication assisted treatment, and testosterone/androgen medications which are sometimes used for gender affirming care.
Medicaid Unwinding Tracker - KFF’s Medicaid Unwinding Tracker provides timely updates monitoring state enrollment and unwinding data and national enrollment data. As of the time of this writing, more than 9.5 million people have been disenrolled from Medicaid programs based on data provided by all 50 states and the District of Columbia. Variation in disenrollment ranges from 66% in Texas and 11% in Illinois. Overall, 72% of disenrollments are due to procedural reasons, rather than qualification reasons, with children accounting for about 39% of those persons disenrolled.
Georgia Reinstates Medicaid Coverage for Thousands Who Got Kicked Off After Error in Renewals - After Georgia was notified by the Centers for Medicare and Medicaid Services (CMS), along with 29 other states, that the state was improperly disenrolling beneficiaries from their Medicaid rolls during the state’s unwinding period, officials from the state began re-enrolling beneficiaries back into the program. Thus far more participants have seen their Medicaid coverage renewed from these ‘ex parte’ renewals than have those seen their coverage continue due to turning in forms. The state expects about 3 million people to go through the renewal process. The sheer volume has proven a challenge. Georgia’s unwinding plan has the state continuing into next year.
Previously: Under Federal Pressure, 30 States Curtail Medicaid Unwinding - In September 2023, CMS told some 30 states to pause and reverse some of their disenrollment activities due to non-compliance with federal rules regarding "‘unwinding’ period (and some standard rules meant to protect Medicaid beneficiaries). CMS estimated some 500,000 people who lost Medicaid or Children’s Health Insurance Program coverage because of problems with their states’ activities rather than due to any authentic issue of eligibility. The U.S. Department of Health and Human Services (HHS) identified these issues were rooted in each of the states’ (and the District of Columbia’s) “auto-renewal” processes. Each were required to stop procedural disenrollments until they could promise otherwise eligible persons would no longer be inappropriately disenrolled.
Plaintiffs Push Back in Fight over Florida’s Medicaid Unwinding - Patient-plaintiffs in a lawsuit in Florida seeking to challenge the state’s disenrollment process, particularly what they argue is a confusing notification letter, and asked for a preliminary injunction and for the judge to order the state to reinstate coverage for some 800,000 people who had lost coverage thus far in the state’s unwinding. Plaintiffs argued their due process rights were infringed upon due to the confusing nature of the letter and that other similarly situated people were at risk of losing coverage while seeking appeal. Attorneys for the state rebuffed these claims arguing the state’s Medicaid program couldn’t shift so quickly, despite repeated notices and requirements from the federal government that the state do just that.
COVID-19 Pandemic Altered Some Parents’ View of Routine Childhood Vaccines - A study published in Vaccine found that parental beliefs and hesitancy over COVID-19 vaccines have spilled over into routine childhood vaccinations. Due to vaccine misinformation rising (and thus hesitancy) there has been a decrease in childhood vaccine uptake and a rise in vaccine-preventable childhood illnesses. The study found that, of the 310 participants, 11% felt vaccines were less safe since the beginning of the pandemic, 12% felt childhood vaccines were less important, and 13% thought childhood vaccines were less effective. Perception of “low” vaccinations rates correlated with a higher rate of belief that childhood vaccines were less effective. researchers also fond that parents who had received their COVID-19 vaccine were more likely to ensure their children were vaccinated with routine childhood vaccines. However, parents who had negative experiences with COVID-19 infections, like hospitalization or severe disease, were less trusting of regular childhood vaccines. While researchers point toward this being evidence of a “pandemic attitude spillover,” the “reverse” may be true as well - parents more trusting of vaccines in general may seek novel vaccines for themselves and their children and those less trusting in vaccines - especially their own childhood vaccines - may not trust newer vaccines. There was evidence of “geographical clustering”, which might be used in future activities to identify areas and communities needing additional outreach, education, and - in the absence of community uptake - additional medical resources.
2% of Kids and 7% of Adults Have Gotten the New COVID Shots, US Data Show - A national survey of thousands of Americans, performed by the Centers for Disease Control and Prevention (CDC), found just about 7% of adults and 2% of children have gotten their updated COVID vaccines for this season. The data also showed some 40% of adults said they “probably” or will “definitely” not get an updated shot. Similar percentages of parents said the same about vaccinating their children. despite cases remaining low relative to the earliest months of the COVID pandemic, about 18,000 people are hospitalized and 1,200 people still die every week from in the United States due to COVID. Novavax, the only non-MRA COVID-19 vaccine in the US market, utilizing a more “traditional” protein based delivery system, is not yet approved for children under the age of 12.
Related: ‘Waiting with Bated Breath’: Health Clinics Anxious for COVID Vaccines Weeks After Rollout - The latest COVID-19 vaccines ran into a bumpy rollout with many consumers waiting in line at their local pharmacies only to be told no stock was available or not even being able to get a hold of a pharmacist in order to check. Similarly, community health centers are reporting having only received a fraction of the doses they ordered while others report not having gotten theirs at all.
8. LATEST NEWS
Advocacy Groups Call on Congress to Reauthorize Global HIV Initiative - A bipartisan alliance of more than 30 health care groups and policy experts are urging Congress to reauthorize the President’s Emergency Plan for AIDS Relief, or PEPFAR, for another 5 years after the program lapsed at the end of September 2023. The fight to reauthorize PEPFAR is now months long with President George W. Bush weighing in and working the Congress with his surrogates. Opposition to PEPFAR appears to originate from a Heritage Foundation report suggesting that PEPFAR is “cover” for the U.S. to send foreign aids money to fund abortions or LGBTQ advocacy and, more abhorrently, stating that “HIV is a lifestyle illness”. remaining dollars in the program should let it last until the end of the 2024 fiscal year. But uncertainty, regardless of any short term funding agreement, would be disasterous for the program credited with saving some 25 million people since its inception.
New WHO Guidance on HIV Viral Suppression and Scientific Updates Released at IAS 2023 - At a presentation at the International AIDS Society Conference in Australia this year, the World Health Organization (WHO) shared updated guidance regarding HIV “suppression” and the likelihood of transmission during sex. Previous guidance focused on “undetectable”. The WHO’s guidance established “suppression” (yellow light) at 1000 copied/ml which is generally higher than the 200 “undetectable” status behind “undetectable = untransmittable” (green light). The “red light” would “unsupressed” or anything over 1,000 copies/ml. The idea reflects the real world experiences of not necessarily being able to access a viral load test readily but being able to assess specificity and sensitivity with rapid tests but also a priority on identifying when a patient might need help with adherence, rather than a lecture. Before giving the guidance change the nod, researchers reviewed studies as far back as 2000 in order to identify a measurable risk of transmission when a viral load is between 600-1000. They found two cases, amounting to a 0.6% observed transmission rate. The guidance does not necessarily “translate” for prevention of mother-to-child transmission.
Court Strikes Down Trump-Era Rule that Allowed Insurers to Not Count Co-Pay Assistance - In early October 2023, HIV + Hep Institute and a group of Diabetes advocacy organizations won a lawsuit against HHS striking down a Trump-era law which allowed insurers to “double-dip” on manufacturer patient assistance programs. The rule allowed insurers to not count the value of manufacturer patient assistance programs against a patient’s deductible or out-of-pocket, even if the insurers sought to capture the full value of the assistance program. The ruling now means only branded medications with a generic equivalent may see such practices and only if that state’s law allows the design to exist.
World Needs to Dramatically Scale Up Hepatitis Testing and Treatment - Viral hepatitis is likely to be a more lethal killer than malaria, tuberculosis, and HIV by 2040, according to the World Health Organization WHO), if current trends are not sufficiently reversed. Testing remains inadequate with just 21% of people living with HCV being diagnosed and just 13% treated (and cured). The WHO argues sufficiently upscaling and streamlining these outreach, education, testing, and linkage to care needs are required in order to reverse these trends - with the goal of eliminating viral hepatitis by 2030 largely falling out of sight for the globe.
Tennessee Hit by Federal Lawsuit Over Discriminatory HIV Sex Worker Statutes - The American Civil Liberties Union and Transgender Law Center have come together to represent OUTMemphis, an LGBTQ+ advocacy and support group for PLWH, and four individuals living with HIV who say they suffered “discriminatory hardship” following convictions for “aggravated prostitution” - which caused them to have to register as “violent” sex offenders for life. Sex work alone in Tennessee is currently only a misdemeanor. However, if the sex worker in question happens to also be a person living with HIV, then the crime is considered “aggravated prostitution”. The plaintiffs argue that they face discriminatory treatment due to a health condition which likely violates the Americans with Disabilities Act (ADA) and that such extreme enhancements are a violation of their 8th and 14th Amendment rights. To our knowledge, this is the first lawsuit citing the ADA which will challenge HIV criminalization laws.
Rulemaking on Older Americans Act Targets Seniors Who are LGBTQ, Living with HIV - In a final rule expected next year for the Older Americans Act, advocates urged the U.S. Department of Health & Human Services (HHS) to adopt explicit inclusion of protections for LGBTQ+ people and people living with HIV. The Older Americans Act is also up for reauthorization next year. Aaron Tax, of SAGE, said the rule would simply require states to do outreach to older LGBTQ people, collect data on their needs, and to collect data on whether they are meeting their needs.” New regulations for the OAA have not been issued since 1988.
How the ADA Paved the Way for Workplace Protections for Women and LGBTQ+ People - Bragdon v Abbott is largely considered a cornerstone ruling regarding the Americans with Disabilities Act (ADA), whichfocused on protecting the access to care for an “asymptomatic” person living with HIV when a dentists refused to fill a cavity for them in the 1990’s. Taking signals from the broad reach of the ADA, and the then-novel concepts used to employ it, civil rights laws and amendments to the ADA are working to carve a more fair societal environment for those who have historically faced discrimination. “It’s part of the non-discrimination mandate that you have to make these modifications simply to create equal opportunity - not give somebody an advantage,” said Ben Klein in an interview with 19th News. Klein is now the senior director of litigation at GLAD but prior to that, he argued on behalf of Sidney Abbott, the patient in the aforementioned case. From pregnancy in the workplace to understand gender dysphoria as a medical condition covered under the ADA, 19th News leverages a historical look at the ADA and a prescient interview with Klein.