The Only Thing That Stays The Same… Welcome to 2026
I want to personally thank those of you who joined me in finishing the sentence that is this week’s blog title.
For CANN and many of our colleagues and partners, 2025 was indeed the year of significant change. From government institutions being shook to their core—a near unprecedented challenge to our societal agreements on “fact”—to our dedication to issues and community through education and growth, who we are today is not who we were on January 1, 2025. And, despite what reflection of harder and more stressful moments might tell us, that change is not necessarily a bad thing.
2026 greets CANN, as an organization, with our own evolution: even as we seek to evolve the policy landscape and our partners to be patient-focused in an ever-increasingly tangible way.
CANN’s growth means permanent work for some of our contractors, allowing for incredible investments in three astounding individuals and their continued development as trend setters and thought leaders. Along with conversion to employee status, Ranier and Kalvin’s roles are expanding to leverage their state-based successes and networks to include federal policy education and advocacy. Travis’s expanded position will find him focused on ensuring CANN’s image is uniform, polished, and properly presented to the world at large. Our friends at Perry Communications are continuing as collaborators in shaping the advocacy ecosystem as patient-driven without fear or favor to the influences impacting access to care.
Our community investments are also growing and evolving in exciting ways. Later this year, CANN will share details on our re-vamped Community Roundtable events and how the “PDAB Summit” will transform into a broader conversation on patient access, drug pricing, and necessary reform and regulation. This discourse will offer real solutions that serve patients rather than simply catering to the next round of shady middlemen or market manipulating investors.
Growth and change is never all easy. The nature of evolution often demands the…unfamiliar.
After twelve years of service to CANN, Brandon Macsata will be moving on to focus on his work with ADAP Advocacy and PlusInc. After coming on to assist CANN’s founder, Bill Arnold, with restructuring the organization in 2014, Brandon continued to shepherd the organization through Bill’s death, including recommending me to the Board as successor CEO. Brandon’s contributions to CANN are immeasurable and we’re grateful for his care in helping us get to here.
This year is CANN’s 30th anniversary and much like any person turning thirty years old, we’ve been steadily evaluating our role, our knowledge, and our power to positively effect change. In 2025, we issued four $10,000, low-barrier advocacy grants to state-based and national partner organizations working on our shared issues from different perspectives. These organizations, Lupus Colorado, Aging and HIV Institute, Pharmacists United for Truth and Transparency, and Epilepsy Alliance of Louisiana, share our mission to define the issues impacting patient access to care across all health conditions and regardless of a patient’s socio-economic status, race, sex or gender, religion, geography, or their health history. We learned from these organizations about services they offer, treatment development pipelines, perspectives on navigating care coverage across programs, challenges in keeping local pharmacy doors open, and how providers, even in schools, can refuse to administer life-saving medication to a child experiencing a seizure simply because they are uncomfortable with the medication determined to be the standard of care. We shared our perspectives on messaging and leveraged our experience in HIV exceptionalism to navigate policy change and demand equitable care for our shared communities.
Because the reality is that HIV is losing its exceptional nature in the policy realm. While our programs remain alive—and, frankly, as of yet still funded—our support systems and our integration into broader, more generalized care as a manner of ensuring actual access for people living with HIV (those systems and changes for which we’ve advocated), are under threat by short-sighted, single-issue discussions. Advocacy organizations who have collected money over the years without innovation or growth will continue to labor without producing results and at the expense of true change-makers. Those who expect to proceed according to their own status quo, mired in bureaucracy, the successes of the past, and without authentic coalition-building will atrophy. Again, that’s authentic coalition-building: as I’ve shared over the years, “we must find friends”. And I don’t mean transactional friends (i.e., “If you sign this letter, I’ll sign yours”), but rather real relationships built in a slower manner that develops attachment without expectation of specific action, and instead deep enough care to show up when a call is made.
This goes beyond our community partners. Our industry partners are facing much the same conversation about the therapeutic areas they invest in and their alliances with advocates. What does it mean to be more than funders? What does it look like to fundamentally transform the patient advocacy ecosystem through stabilizing investments? What does it mean to support incubator models of advocacy? How does this impact or otherwise become integrated into a company’s business model? The answers to these questions are not static and they are far beyond any quarterly stockholder assessment, and our partners know this.
Advocates, for our parts, must be responsive to a rapidly-changing environment where those most confident in their proposed “solutions” are only having half the conversation on a single-issue within a larger web of issues, networked in a complex chain reaction of impacts. The consequences of policymakers—state and federal—suffering from “tunnel vision” or engaging in cheap, inaccurate, and deceptive talking points are manifesting in readily foreseeable conflicts making their way through expensive and unnecessary legal battles at the expense of tax dollars and patient quality and affordability of care.
We can do better than this. As HIV advocates, we know how to leverage historical challenges into future successes. We know how to seek out the unforeseeable and to respond to clever callousness. We have an obligation to share this knowledge and empower partners across the country to achieve what we have and, in doing so, to save our own investments from today’s threats.
2026 requires a paradigm shift from institutional comfort to nimble, responsive, powerful advocacy.
The changes 2025 brought us will be the continued challenges of 2026. To which the answer must be “We can.” (…CANN)
How Lupus Colorado and CANN Built an Advocacy Powerhouse
Some partnerships start with a formal strategy session. Ours began with a simple realization. People living with lupus and people living with HIV or viral hepatitis face many of the same obstacles when they try to get the care they need. Once we recognized that shared landscape, the connection between Lupus Colorado and the Community Access National Network (CANN) started to feel not only natural, but necessary.
CANN’s mission is to define, promote, and improve access to healthcare services and supports for people living with HIV and people living with viral hepatitis. The focus is on keeping care affordable and within reach for people no matter where they live, what they earn, or how they are insured. As soon as we spent time together, it was clear how much that mission speaks to the lived reality of people living with lupus. Many in our community depend on the same safety net programs, the same specialty pharmacies, and the same policies that protect medication access for people with chronic conditions nationwide.
Once we began sharing stories, the overlap came into full view. We heard about people living with lupus who rely on the 340B Drug Pricing Program to keep their treatment affordable. We heard about people managing both lupus and other long term conditions who face the same hurdles that people living with HIV or hepatitis encounter when insurance rules change or formularies shift. We also heard how uncertainty about programs like the 340B Program or state efforts to set Upper Payment Limits through bodies like the Colorado Prescription Drug Affordability Board creates stress and confusion for everyone who counts on steady access to treatment. The policy language may sound technical, but the effects show up in daily life. That shared experience became the spark that brought our organizations together.
Lupus Colorado eventually became a kind of testing ground for patient engagement around these issues. When debates surrounding 340B and the state board intensified, we invited our community to learn more and speak up. People living with lupus stepped forward with thoughtful questions about what these changes might mean for their medications and their stability. They shared stories about years spent finding the right treatments. They talked about the worry that comes with not knowing whether a familiar pharmacy or discount program will still be available. Their honesty helped shape a clearer picture of what access really means for people with chronic conditions.
CANN supported this work by offering context, training, and a strong national network. Their experience showed us how these local conversations fit into broader public health goals, including ongoing efforts to build sustainable systems for people living with HIV and long term strategies for hepatitis C elimination. They also helped amplify our stories by connecting them to advocates, policymakers, and public health leaders who are working to protect and expand medication access nationwide.
As we continued working together, the partnership grew into something that felt like friendship and, at times, even family. It surprised all of us how quickly Louisiana and Colorado began to feel connected through shared purpose and shared energy. This work is deeply personal, and when good people come together with the belief that collaboration, not competition, brings out our strongest work, something close to magic begins to take shape. My curiosity and strategic thinking blended naturally with Jen’s mentorship and policy insight. Kalvin and Ranier added necessary context for any variety of issues we shared and even accompanied me to some meetings to ensure Lupus Colorado's interests were well-defined for our audience. Together we created a combination that strengthened every effort that followed.
This collaboration also changed how advocacy feels for many people. Instead of seeing policy as something decided far away, our community began to see it as something they can influence. When people speak about their lives, policymakers listen differently. The technical language becomes human. The stakes become easier to understand. And the entire conversation shifts toward solutions that protect access rather than limit it.
The partnership between Lupus Colorado and CANN has shown us that when organizations connect around shared purpose, everyone benefits. We bring different histories and different areas of expertise, but we are united by our commitment to ensure that people living with chronic conditions can access the care they need without fear or financial hardship. Our collaboration continues to strengthen advocacy networks, uplift patient voices, and support progress toward public health goals that matter to all of us.
Most of all, this partnership reminds us that no one faces this journey alone. When communities work together, even unlikely collaborations can grow into something powerful enough to spark change.
Antibiotic Crisis: Hope Amid Institutional Decline
The fight against antimicrobial resistance (AMR) in sexually transmitted infections (STIs) stands at a critical crossroads. Despite a slight decline in U.S. STI rates in 2023, resistance to antibiotics continues to rise globally, particularly for gonorrhea. This creates a paradox: while we're seeing promising new treatments advancing toward approval, recent political decisions have dramatically weakened our ability to track resistance patterns. Meanwhile, funding and policy support for developing new antibiotics remain inadequate. This crisis demands urgent attention as resistant infections spread faster than new treatments can be developed, with serious implications for public health and patient care.
The Growing Threat of Antibiotic-Resistant STIs
For people living with chronic conditions or compromised immune systems, antibiotic-resistant STIs aren't just a public health statistic—they represent a serious and growing threat to wellbeing. While the Centers for Disease Control and Prevention (CDC) reported fewer gonorrhea cases in the U.S. last year, the global picture is far more concerning.
Gonorrhea is becoming increasingly resistant to our last effective treatments. In Southeast Asia, the World Health Organization (WHO) identified a three-fold increase in extensively drug-resistant gonorrhea strains in Cambodia between 2022 and 2023. These hard-to-treat infections now make up over 12% of cases in the region.
What does this mean for patients? When first-line treatments fail, people face longer infectious periods, more complex and expensive treatments, and greater risk of complications. For people living with HIV or hepatitis C, these resistant infections can further compromise health and complicate disease management.
Breakthrough Treatments on the Horizon
Despite the grim outlook, two novel antibiotics represent genuine breakthroughs in the fight against resistant STIs after decades without new gonorrhea treatments.
Zoliflodacin, developed through a Global Antibiotic Research & Development Partnership (GARDP) and Innoviva Specialty Therapeutics partnership, completed the largest Phase 3 trial ever conducted for gonorrhea, with promising results. While its 91% cure rate appears slightly lower than the current standard's 96%, zoliflodacin's significance lies in its novel mechanism of action against resistant strains and its oral administration route. As resistant gonorrhea increasingly requires injectable treatments, an effective oral option represents a major advance for both accessibility and patient care.
Equally promising is gepotidacin, developed by GSK and already FDA-approved for urinary tract infections as of March 2025. This novel antibiotic showed a 92.6% success rate against gonorrhea through its unique dual-targeting mechanism that inhibits two critical bacterial enzymes, making it effective against resistant strains. GSK plans to submit for the gonorrhea indication later in 2025.
These developments showcase complementary partnership models: GARDP's non-profit approach ensures zoliflodacin's availability in low-income countries, while gepotidacin demonstrates successful public-private partnership between GSK and BARDA. Despite these advances, the WHO reports the broader antibiotic pipeline remains critically thin, with only 12 truly innovative antibiotics among 32 in development, and just 4 targeting the most critical pathogens.
Political Decisions Undermining Public Health
In a dangerous contradiction, just as resistance is rising and new treatments are on the horizon, political decisions have severely weakened our ability to monitor and respond to these threats.
Since early 2025, the current administration has eliminated approximately 20,000 jobs across health agencies and proposed cutting the HHS budget by about 26% ($127 billion).
The impact on STI programs has been particularly severe. The Washington Post reported that all 27 scientists at the only U.S. facility capable of tracking hepatitis outbreaks were fired. Additionally, 77 CDC staff members working on STI prevention were let go, including 49 experts embedded in state health departments who provided critical support to local efforts.
Most alarming for people at risk of STIs is the closure of the specialized lab that tests gonorrhea samples for antibiotic resistance. This lab was our early warning system—without it, doctors and patients won't know which antibiotics still work until treatment failures start mounting.
Prevention Strategies: Interrupting Transmission Chains
While developing new antibiotics is critical, prevention remains essential. Doxycycline Post-Exposure Prophylaxis (DoxyPEP) has emerged as an effective tool for breaking transmission chains. The CDC now recommends that men who have sex with men and transgender women with a history of bacterial STIs use DoxyPEP after sexual encounters.
Real-world data from San Francisco showed significant declines in chlamydia and syphilis among those using DoxyPEP, though gonorrhea reductions were less dramatic. While some concerns exist about the potential for DoxyPEP to contribute to broader antibiotic resistance, current evidence suggests this approach can effectively reduce STI transmission in high-risk groups—a crucial tool while we wait for new treatments.
The Funding Gap and Market Failure
The fundamental problem in antibiotic development is an economic one: the market doesn't adequately reward the creation of new antibiotics, especially those held in reserve to combat resistance.
The experiences of both zoliflodacin and gepotidacin highlight this challenge. Zoliflodacin required non-profit involvement through GARDP to advance through clinical trials, while gepotidacin needed significant government funding through BARDA. As Henry Skinner of the AMR Action Fund notes, "The funds needed to support this ecosystem, particularly in late-stage development, won't be there in a couple of years unless something unanticipated happens."
The AMR Action Fund, backed by pharmaceutical companies, aims to invest $1 billion to bring 2-4 new antibiotics to patients by 2030. The Fund has deployed over $100 million in capital to companies developing promising antimicrobials. However, experts recognize this as a stopgap measure rather than a solution to the underlying market failure.
A more sustainable approach is proposed in the PASTEUR Act, which has been introduced in multiple congressional sessions without passing. This legislation would create subscription contracts with developers of critical antimicrobials, ensuring financial returns regardless of how sparingly the drugs are used—essentially paying for access rather than volume.
This "Netflix model" for antibiotics would help align public health needs with market incentives. However, despite bipartisan support, the Act faces an uncertain future in the current political climate of budget cutting and deregulation.
Disproportionate Impact on Vulnerable Communities
Antimicrobial resistance operates within complex syndemics, where multiple health conditions interact and amplify each other within populations experiencing social inequities. People living with HIV stand at the intersection of these overlapping epidemics.
Research shows people living with HIV have higher rates of drug-resistant gonorrhea co-infection, each condition worsening the other. This syndemic intensifies with hepatitis C—a Department of Veterans Affairs study found 37% of people with HIV were also HCV-positive, with significantly higher rates of mental health issues and substance use disorders among these co-infected patients.
Among people who inject drugs with HIV, HCV rates reach up to 71% in some settings, according to a global review. These aren't coincidental occurrences—structural factors create environments where these epidemics cluster and interact.
The dismantling of surveillance infrastructure creates a dangerous blind spot in tracking these syndemics. Without specialized CDC labs monitoring resistant gonorrhea, we've lost our early warning system for emerging resistance patterns in vulnerable communities. Simultaneously, new restrictions on health equity research effectively discourage scientists from studying social factors that increase vulnerability to antimicrobial resistance.
A Patient-Centered Path Forward
From a patient and advocate perspective, five key policy areas require immediate attention:
Restore critical infrastructure. The dismantling of STI surveillance labs has left both patients and providers flying blind. Congress must fund restoration of these capabilities and hold administration officials accountable so we can track resistance patterns, update treatment guidelines, and support state and local health departments.
Support innovative development models. The GARDP partnership for zoliflodacin and the GSK-BARDA collaboration that produced gepotidacin demonstrate effective approaches to antibiotic development. These models—balancing commercial viability with public health needs—warrant expanded funding and replication.
Implement pull incentives. The PASTEUR Act would create a subscription-based model rewarding companies for developing critically-needed antibiotics without encouraging overuse, aligning market incentives with public health priorities.
Strengthen integrated care models. People at highest risk of resistant infections often face multiple health challenges. HIV, HCV, and STI services should be integrated to address overlapping needs, following the Ryan White HIV/AIDS Program's comprehensive care model.
Expand prevention strategies. While new treatments are essential, preventing infections reduces suffering and limits resistance. Expanded access to DoxyPEP, increased STI screening in high-risk populations, and vaccine research represent critical prevention strategies.
The antimicrobial resistance crisis in STIs reveals a stunning act of self-sabotage: just as scientific innovation finally delivers promising new treatments like zoliflodacin and gepotidacin, the misguided decimation of public health infrastructure has crippled our ability to track and respond to resistant infections. This isn't poor timing—it's the cavalier dismemberment of critical surveillance systems by ill-equipped partisans wielding policy chainsaws with no regard for consequences. The resulting wreckage threatens to undo decades of progress against STIs, particularly for communities already navigating systemic barriers to care.
The path forward demands both hope and principled outrage. Patients and advocates must forcefully reject further cuts to public health infrastructure, demand immediate restoration of STI surveillance capabilities, and hold elected officials accountable for the consequences of their decisions. We must insist on passage of the PASTEUR Act to fix the broken economics of antibiotic development while ensuring that promising science reaches those who need it most, not just those with wealth, power, and access.
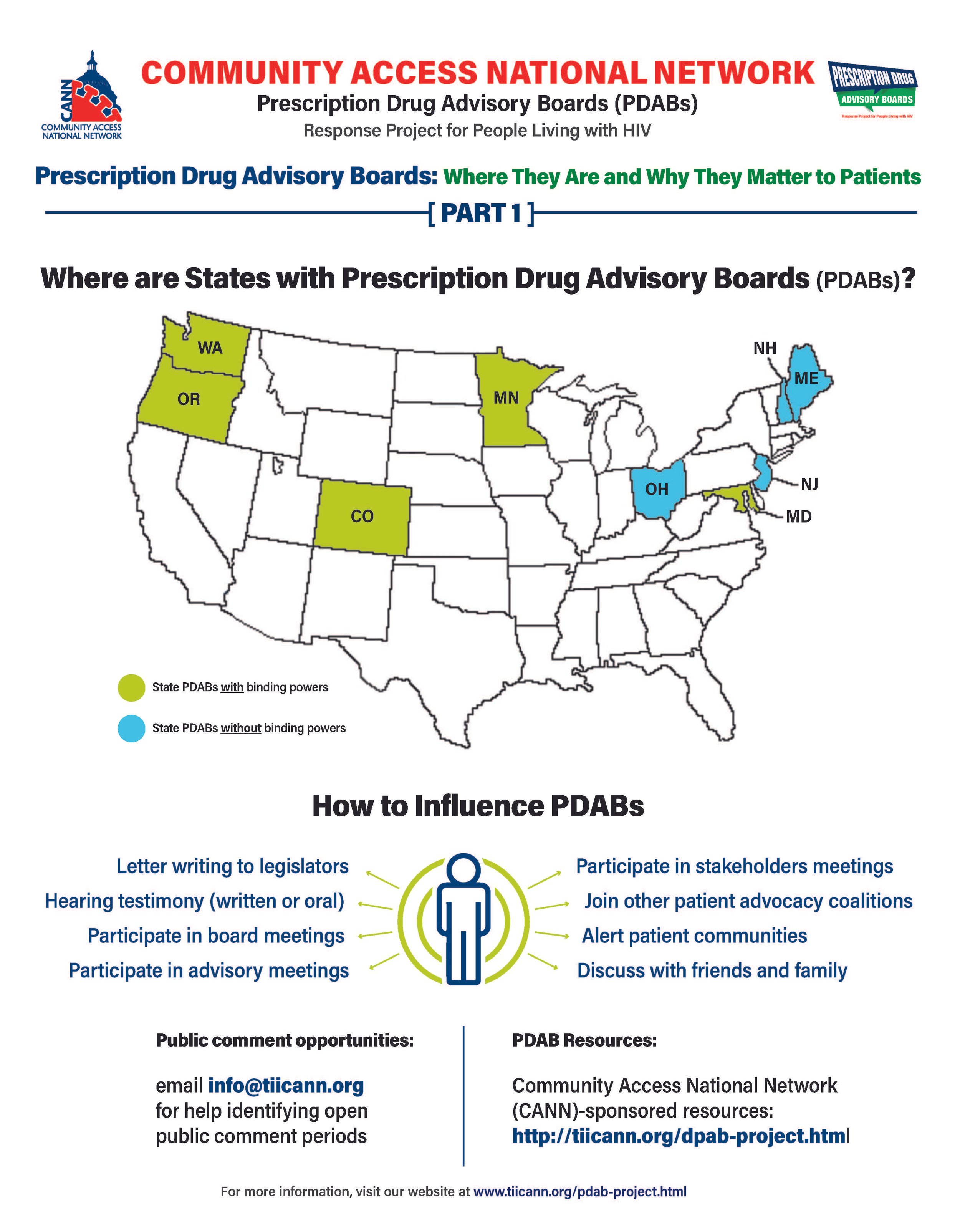
PDAB Chicanery: How Drug Affordability Boards Are Undermining Public Engagement
Prescription Drug Affordability Boards (PDABs) across the country are playing a dangerous game with public engagement—one where they keep changing the rules and moving the goalposts. From inadequate notice periods to last-minute document releases, these boards are creating barriers that echo troubling federal trends, effectively sidelining the very people who have the most at stake: patients.
These state-level games mirror concerning federal developments, most notably the rescinding of the Richardson Waiver by U.S. Department of Health & Human Services (HHS) Secretary Robert F. Kennedy, Jr. This action removed a 50-year precedent requiring public input on HHS rules—effectively telling patients and advocates their opinions aren't welcome at the policy table.
As these transparency rollbacks continue, people who rely on medications face increasing uncertainty about their access to life-sustaining treatments—while boards claim to represent their interests through processes that actively exclude them.
Maryland PDAB: How to Follow the Letter of the Law While Breaking Its Spirit
Maryland's Prescription Drug Affordability Board offers a master class in technical compliance that functionally blocks meaningful public participation. Their recent meeting preparation tactics exemplify how these boards can check procedural boxes while effectively sidelining patient voices.
On March 18, 2025, the Maryland PDAB posted a revised agenda for their upcoming March 24 meeting. This might seem unremarkable until you realize the public comment deadline was March 19—giving stakeholders exactly one day to review, analyze, and formulate responses to complex pharmaceutical policy documents. The revised agenda wasn't a minor update either. It contained material differences from the previous version, including a comprehensive cost review dossier for Farxiga, a medication critical for many people with diabetes and heart failure.
As CANN's letter to the board noted, "Posting the updated agenda with associated meeting materials the day before the deadline for comment is not a good faith effort in garnering public trust, nor does it display value in public input." The Maryland PDAB's approach creates a veneer of public engagement while practically guaranteeing that meaningful input will be minimal.
This pattern suggests the board views public comment as a procedural hurdle rather than a valuable source of insight. By technically fulfilling their obligation to post materials before the comment deadline (even if by mere hours), they've found a convenient loophole that undermines the very transparency standards that public notice requirements are designed to uphold.
The Maryland case isn't an anomaly. It's a symptom of a growing tendency to treat public engagement as an inconvenient formality rather than a crucial component of sound healthcare policy development.
The Federal Parallel: HHS and the Richardson Waiver
The state-level PDAB maneuvers don't exist in a vacuum. They mirror a troubling federal precedent set by HHS Secretary Robert F. Kennedy, Jr., who recently rescinded the Richardson Waiver—a decision that effectively slams the door on patient advocacy at the federal level.
The Richardson Waiver has a 50-year history. Established in 1971, it required HHS to subject matters relating to "public property, loans, grants, benefits, or contracts" to the American Procedures Act's notice and comment rulemaking guidelines. This waiver was created specifically to ensure public voices would be heard on matters that directly affect their health and well-being.
Now, that protection is gone. The new HHS rule claims the waiver "impose[s] costs on the Department and the public, are contrary to the efficient operation of the Department, and impede the Department's flexibility to adapt quickly to legal and policy mandates." This bureaucratic language translates to a simple message: we don't care what you think.
God forbid they remember who they work for.
And the impact is far-reaching. While Medicare remains protected under separate provisions of the Medicare Act, critical programs like Medicaid, SAMHSA, and the Administration for Children and Families now operate without mandated public comment periods. Legal experts note this could allow for swift implementation of controversial measures like Medicaid work requirements without going through normal rulemaking processes.
The timing is particularly ironic given the Office of Management and Budget's recent guidance letter emphasizing the importance of "broadening public participation and community engagement" and making it "easier for the American people to share their knowledge, needs, ideas, and lived experiences to improve how government works for and with them."
This federal retreat from transparency sets a dangerous tone that state-level boards appear eager to follow.
Other State PDAB Examples: Oregon and Colorado's Concerning Patterns
Maryland isn't alone in its questionable approach to public engagement. Oregon's PDAB recently decided to include Odefsey—an antiretroviral medication for people living with HIV—on its list for cost control exploration, contradicting previous discussions to protect these medications. While they claim they might reconsider based on affordability research, this flip-flop creates unnecessary anxiety for people who depend on these treatments.
Colorado's PDAB situation is particularly egregious. Since 2023, CANN has repeatedly requested that the board consult with the state health department about rebate impacts on public health infrastructure and patient affordability—concerns echoed by the former SDAP director and PDAB members themselves.
Yet Colorado PDAB staff have consistently avoided conducting a proper fiscal impact analysis, bluntly stating "We won't be doing that" when asked directly. This refusal persisted even as formal rulemaking began, which triggers statutory requirements for analyses under Colorado's Administrative Procedure Act.
The board has repeatedly postponed its first rulemaking hearing, effectively delaying compliance with transparency requirements. Meanwhile, the Joint Budget Committee has begun questioning the PDAB's financial accountability, receiving only partial responses about consultant costs and litigation expenses.
Most concerning is the disconnect between PDAB actions and demonstrated patient benefits. A 2024 analysis of Oregon's similar program showed states would need additional funds to maintain programs under an upper payment limit system—with no meaningful patient affordability improvements identified.
Patient Impact: Why This Matters
Behind the procedural games and policy maneuvers are real people whose lives hang in the balance. The Colorado PDAB's actions exemplify how these bureaucratic decisions create genuine fear and uncertainty for people with rare diseases and conditions requiring specialized medications.
Twelve-year-old Avery Kluck lives with Aicardi syndrome and faces life-threatening seizures that have been intensifying. Her doctors recommended Sabril, a powerful anticonvulsant costing up to $10,000 per month—a medication on Colorado's PDAB radar for potential price controls.
"We're to a point now where her seizures are getting more violent, and this is our last resort," explains Heather Kluck, Avery's mother. "And now I'm finding out she may not have access to it." The family faces an impossible choice between starting a medication that might become unavailable or watching their daughter suffer.
This uncertainty isn't theoretical. At least one pharmaceutical company has already threatened to pull drugs from Colorado if price caps are imposed. For medications like Sabril, which are dangerous to discontinue abruptly, such market exits could be catastrophic.
People living with cystic fibrosis also had to mobilize to prevent Colorado's PDAB from declaring Trikafta "unaffordable," with one parent describing the experience as "torturous for our family" and another stating: "It's an experiment, and it's really gross that they're doing it on people who are really sick."
The irony is painful: boards created to increase medication access may end up restricting it for those who need it most.
Conclusion
These boards, created under the guise of helping patients afford medications, are operating in ways that actively silence patient voices. From Maryland's last-minute document dumps to Colorado's refusal to conduct impact analyses and Oregon's policy reversals on critical medications, these boards are erecting barriers that exclude the very people who will bear the consequences of their decisions.
The problems run deeper than procedural failures. The fundamental approach of PDABs—attempting to control drug prices without adequately assessing impacts on patient access—risks creating catastrophic unintended consequences for people who depend on specialized medications. Avery Kluck and others living with rare conditions don't have the luxury of waiting while boards experiment with price controls that might make their life-saving treatments unavailable.
The pattern is clear: from the federal level with RFK Jr.'s dismantling of public comment protections to state PDABs playing administrative games, we're witnessing a coordinated retreat from meaningful public engagement in healthcare policy. This isn't just bad governance—it's dangerous for patients.
States should seriously reconsider whether PDABs serve any legitimate purpose beyond political theater. At minimum, stakeholders across the healthcare spectrum must demand that these boards either implement truly transparent, patient-centered processes or acknowledge they cannot fulfill their stated mission without causing harm to the very people they claim to help.
Flying Blind: Public Health Without Population Data
On January 31, 2025, federal health agencies began removing thousands of webpages and datasets from public access in response to executive orders from the Trump Administration targeting "gender ideology" and diversity, equity, and inclusion initiatives. By February 1, over 8,000 federal webpages and 450 government domains had gone dark, including critical public health resources from the Centers for Disease Control and Prevention (CDC), National Institutes of Health (NIH), and Food and Drug Administration (FDA).
Immunologist and microbiologist Dr. Andrea Love, Executive Director of the American Lyme Disease Foundation, minced no words regarding the executive actions: "If you weren't clear: a President ordering a Federal health and disease agency to delete pages on its website is a public health crisis." The scope of removed content spans decades of population health data, from the 40-year-old Youth Risk Behavior Surveillance System to current HIV surveillance statistics. Many pages that have returned now display banners warning of further modifications, creating uncertainty around the future availability and integrity of federal health data.
This sudden removal of public health information echoes similar challenges faced during the early COVID-19 response, when limited access to comprehensive population data hampered the ability to identify and address emerging health disparities. As we examine the current situation, the key question becomes: How can evidence-based public health function without access to the very data that drives decision-making and ensures equitable health outcomes?
Scale of Impact
The removal of federal health datasets represents an unprecedented disruption to public health surveillance and research capabilities. According to KFF analysis, key resources taken offline include:
The CDC's Youth Risk Behavior Surveillance System, which for 40 years has tracked critical health indicators among high school students. This dataset has been instrumental in identifying emerging health crises, including the rise in youth mental health challenges and substance use patterns.
CDC's AtlasPlus tool, containing nearly 20 years of surveillance data for HIV, viral hepatitis, sexually transmitted infections, and tuberculosis, is no longer accessible. This platform has been essential for tracking disease trends and designing targeted prevention strategies.
The Social Vulnerability Index and Environmental Justice Index - critical tools for identifying communities at heightened risk during public health emergencies and environmental disasters - have also been removed. These resources help public health officials allocate resources effectively during crises and natural disasters.
Public health researchers report that the loss of demographic data collection and analysis capabilities particularly impacts their ability to identify and address health disparities.
As Dr. Jennifer Nuzzo, director of the Pandemic Center at Brown University School of Public Health notes, "Health equity is basically all of public health."
The ability to analyze health outcomes across different populations is fundamental to developing effective interventions and ensuring equitable access to care.
The CDC's healthcare provider resources have also been affected, including treatment guidelines for sexually transmitted infections and HIV prevention protocols. This loss of clinical guidance materials creates immediate challenges for healthcare providers working to deliver evidence-based care.
Beyond individual datasets, this wholesale removal of public health information disrupts the interconnected nature of federal health data systems. Many of these resources inform each other, creating compounding effects when multiple datasets become unavailable simultaneously.
Research and Care Delivery Impact
The removal of federal health data creates immediate challenges for both research and clinical care delivery. The Infectious Diseases Society of America (IDSA) warned that removing HIV and LGBTQ+ related resources from CDC websites "creates a dangerous gap in scientific information and data to monitor and respond to disease outbreaks."
This impact is particularly acute in STI prevention and treatment. Including gender and demographic data in research helps identify populations at elevated risk for infections like syphilis, which has reached its highest levels in 50 years. Without this data, developing targeted interventions becomes significantly more challenging.
For HIV prevention specifically, the loss of CDC's AtlasPlus tool removes access to critical surveillance data that guides prevention and treatment strategies. Healthcare providers report that missing CDC clinical guidance on HIV testing and PrEP prescribing creates uncertainty in delivering evidence-based care.
David Harvey, executive director of the National Coalition of STD Directors, emphasizes the immediate clinical impact: "Doctors in every community in America rely on the STI treatment guidelines to know what tests to run, to know what antibiotic will work on which infection, and how to avoid worsening antibiotic resistance. These are the guidelines for treating congenital syphilis, for preventing HIV from spreading, and for keeping regular people healthy every time they go to the doctor."
The loss of demographic data collection capabilities also threatens to undermine decades of progress in understanding and addressing health disparities. Research requiring analysis of health outcomes across different populations may face delays or compromised results without access to comprehensive federal datasets.
This disruption extends beyond immediate clinical care to impact long-term research projects and clinical trials. FDA guidance documents about ensuring diverse representation in clinical studies are no longer accessible, potentially affecting the development of new treatments and their applicability across different populations.
Historical Context and Implications
The current removal of federal health data follows concerning precedent. During the COVID-19 pandemic, similar actions to restrict access to public health data hampered effective response. In July 2020, hospital COVID-19 data reporting was moved from CDC control to a private contractor, leading to significant gaps in data access and accuracy that impeded pandemic response.
As Harvard epidemiologist Nancy Krieger notes, "There's been a history in this country recently of trying to make data disappear, as if that makes problems disappear... But the problems don't disappear, and the suffering gets worse."
This observation proved accurate during COVID-19, when limited access to comprehensive demographic data delayed recognition of disparate impacts on communities of color.
Early COVID-19 response efforts were hampered by insufficient data about how the virus affected different populations. This information gap contributed to delayed identification of emerging hotspots and slowed targeted intervention efforts. The result was preventable disparities in COVID-19 outcomes, particularly among Black, Hispanic, and Native American communities.
Today's wholesale removal of federal health data risks recreating similar blind spots across multiple public health challenges. Without demographic data to identify disparities and guide interventions, public health officials lose the ability to effectively target resources and measure outcomes. As Dr. Jennifer Nuzzo emphasizes, this data is "really important for us to answer the essential question of public health, which is, Who is being affected and how do we best target our limited resources?"
Legal Response and Policy Challenges
On February 4, 2025, Doctors for America filed suit against multiple federal agencies including the Office of Personnel Management (OPM), Centers for Disease Control and Prevention (CDC), Food and Drug Administration (FDA), and Department of Health and Human Services (HHS).
The lawsuit challenges two key actions: OPM's directive requiring agencies to remove webpages and datasets, and the subsequent removal of critical health information by CDC, FDA, and HHS. The complaint argues these actions violated both the Administrative Procedure Act and the Paperwork Reduction Act of 1995 (PRA).
Under the PRA, federal agencies must "ensure that the public has timely and equitable access to the agency's public information" and "provide adequate notice when initiating, substantially modifying, or terminating significant information dissemination products." The complaint alleges agencies failed to provide required notice before removing vital health information and datasets.
The legal challenge emphasizes the fundamental role these datasets play in public health. According to the filing, "The removal of the webpages and datasets creates a dangerous gap in the scientific data available to monitor and respond to disease outbreaks, deprives physicians of resources that guide clinical practice, and takes away key resources for communicating and engaging with patients."
Nine out of twelve public health researchers on CDC's advisory board signed a letter to the agency's acting director seeking explanation for the data removal. These scientists expect to face consequences for speaking out but emphasize the critical nature of maintaining public access to health data.
Data Preservation Efforts
As federal health datasets disappeared, researchers and institutions launched rapid preservation efforts. Harvard University organized its first "datathon" to archive website content through the Wayback Machine, while other academic institutions worked to preserve datasets locally.
The Kaiser Family Foundation reports having downloaded significant portions of CDC data prior to removal. While some CDC data files have been restored, they currently lack essential documentation like questionnaires and codebooks needed for analysis.
For healthcare providers needing immediate access to clinical guidelines, medical associations are working to provide archived copies of treatment protocols. The Infectious Disease Society of America and HIV Medicine Association are coordinating with members to ensure continued access to critical clinical resources.
State health departments maintain some parallel data collection systems that may help fill gaps in federal surveillance. However, these systems often rely on federal frameworks for standardization and analysis, potentially limiting their utility as standalone resources.
These preservation efforts, while necessary, cannot fully replace the coordinated federal data infrastructure needed for comprehensive public health surveillance and research.
Recommendations
Healthcare providers and public health officials should consider these immediate steps to ensure continued access to vital health information:
Data Access and Preservation
Download and securely store copies of restored CDC datasets, including documentation
Maintain offline copies of current clinical guidelines and protocols
Establish relationships with academic institutions archiving federal health data
Alternative Data Sources
Connect with state and local health departments to access regional surveillance data
Utilize medical society and professional organization resources for clinical guidance
Consider participating in alternative data collection networks being established by research institutions
Advocacy Actions
Support ongoing legal efforts to restore data access
Document specific impacts of data loss on care delivery and research
Engage with professional organizations coordinating preservation efforts
Future Planning
Develop contingency plans for maintaining essential health surveillance
Build redundant data collection systems where feasible
Strengthen partnerships with academic and nonprofit research organizations
These steps cannot fully replace federal health data infrastructure but may help maintain critical public health functions while broader access issues are resolved.
When Algorithms Deny Care: The Insurance Industry's AI War Against Patients
The assassination of UnitedHealthcare CEO Brian Thompson in December 2024 laid bare a healthcare crisis where insurance companies use artificial intelligence to systematically deny care while posting record profits. Federal data shows UnitedHealthcare, which covers 49 million Americans, denied nearly one-third of all in-network claims in 2022 - the highest rate among major insurers.
This reflects an industry-wide strategy that insurance scholar Jay Feinman calls "delay, deny, defend" - now supercharged by AI. These systems automatically deny claims, delay payment, and force sick people to defend their right to care through complex appeals. A Commonwealth Fund survey found 45% of working-age adults with insurance faced denied coverage for services they believed should be covered.
The consequences are devastating. As documented cases show, these automated denial systems routinely override physician recommendations for essential care, creating a system where algorithms, not doctors, decide who receives treatment. For those who do appeal, insurers approve at least some form of care about half the time. This creates a perverse incentive structure where insurers can deny claims broadly, knowing most people will not fight back. For the people trapped in this system, the stakes could not be higher - this is quite literally a matter of life and death.
The Rise of AI in Claims Processing
Health insurers have increasingly turned to AI systems to automate claims processing and denials, fundamentally changing how coverage decisions are made. A ProPublica investigation revealed that Cigna's PXDX system allows its doctors to deny claims without reviewing patient files, processing roughly 300,000 denials in just two months. "We literally click and submit. It takes all of 1.2 seconds to do 50 at a time," a former Cigna doctor reported.
The scope of automated denials extends beyond Cigna. UnitedHealth Group's NaviHealth uses an AI tool called "nH Predict" to determine length-of-stay recommendations for people in rehabilitation facilities. According to STAT News, this system generates precise predictions about recovery timelines and discharge dates without accounting for people's individual circumstances or their doctors' medical judgment. While NaviHealth claims its algorithm is merely a "guide" for discharge planning, its marketing materials boast about "significantly reducing costs specific to unnecessary care."
Only about 1% of denied claims are appealed, despite high rates of denials being overturned when challenged. This creates a system where insurers can use AI to broadly deny claims, knowing most people will not contest the decisions. The practice raises serious ethical concerns about algorithmic decision-making in healthcare, especially when such systems prioritize cost savings over medical necessity and doctor recommendations.
Impact on Patient Care
The human cost of AI-driven claim denials reveals a systemic strategy of "delay, deny, defend" that puts profits over patients. STAT News reports the case of Frances Walter, an 85-year-old with a shattered shoulder and pain medication allergies, whose story exemplifies the cruel efficiency of algorithmic denial systems. NaviHealth's algorithm predicted she would recover in 16.6 days, prompting her insurer to cut off payment despite medical notes showing she could not dress herself, use the bathroom independently, or operate a walker. She was forced to spend her life savings and enroll in Medicaid to continue necessary rehabilitation.
Walter's case is not unique. Despite her medical team's objections, UnitedHealthcare terminated her coverage based solely on an algorithm's prediction. Her appeal was denied twice, and when she finally received an administrative hearing, UnitedHealthcare didn't even send a representative - yet the judge still sided with the company. Walter's case reveals how the system is stacked against patients: insurers can deny care with a keystroke, forcing people to navigate a complex appeals process while their health deteriorates.
The fundamental doctor-patient relationship is being undermined as healthcare facilities face increasing pressure to align their treatment recommendations with algorithmic predictions. The Commonwealth Fund found that 60% of people who face denials experience delayed care, with half reporting their health problems worsened while waiting for insurance approval. Behind each statistic are countless stories like Walter's - people suffering while fighting faceless algorithms for their right to medical care.
The AI Arms Race in Healthcare Claims
Healthcare providers are fighting back against automated denials by deploying their own AI tools. New startups like Claimable and FightHealthInsurance.com help patients and providers challenge insurer denials, with Claimable achieving an 85% success rate in overturning denials. Care New England reduced authorization-related denials by 55% using AI assistance.
While these counter-measures show promise, they highlight a perverse reality: healthcare providers must now divert critical resources away from patient care to wage algorithmic warfare against insurance companies. The Mayo Clinic has cut 30 full-time positions and spent $700,000 on AI tools simply to fight denials. As Dr. Robert Wachter of UCSF notes, "You have automatic conflict. Their AI will deny our AI, and we'll go back and forth."
This technological arms race exemplifies how far the American healthcare system has strayed from its purpose. Instead of focusing on patient care, providers must invest millions in AI tools to combat insurers' automated denial systems - resources that could be spent on direct patient care, medical research, or improving healthcare delivery. The emergence of these counter-measures, while potentially helpful for providers and patients seeking care, highlights fundamental flaws in our healthcare system that require policy solutions, not just technological fixes.
AI Bias: Amplifying Healthcare Inequities
The potential for AI systems to perpetuate and intensify existing healthcare disparities is deeply concerning. A comprehensive JAMA Network Open study examining insurance claim denials revealed that at-risk populations experience significantly higher denial rates.
The research found:
Low-income patients had 43% higher odds of claim denials compared to high-income patients
Patients with high school education or less experienced denial rates of 1.79%, versus 1.14% for college-educated patients
Racial and ethnic minorities faced disproportionate denial rates:
Asian patients: 2.72% denial rate
Hispanic patients: 2.44% denial rate
Non-Hispanic Black patients: 2.04% denial rate
Non-Hispanic White patients: 1.13% denial rate
The National Association of Insurance Commissioners (NAIC) Consumer Representatives report warns that AI tools, often trained on historically biased datasets, can "exacerbate existing bias and discrimination, particularly for marginalized and disenfranchised communities."
These systemic biases stem from persistent underrepresentation in clinical research datasets, which means AI algorithms learn and perpetuate historical inequities. The result is a feedback loop where technological "efficiency" becomes a mechanism for deepening healthcare disparities.
Legislative Response and Regulatory Oversight
While California's Physicians Make Decisions Act and new Centers for Medicare & Medicaid Services (CMS) rules represent progress in regulating AI in healthcare claims, the NAIC warns that current oversight remains inadequate. California's law prohibits insurers from using AI algorithms as the sole basis for denying medically necessary claims and establishes strict processing deadlines: five business days for standard cases, 72 hours for urgent cases, and 30 days for retrospective reviews.
At the federal level, CMS now requires Medicare Advantage plans to base coverage decisions on individual circumstances rather than algorithmic predictions. As of January 2024, coverage denials must be reviewed by physicians with relevant expertise, and plans must follow original Medicare coverage criteria. CMS Deputy Administrator Meena Seshamani promises audits and enforcement actions, including civil penalties and enrollment suspensions for non-compliance.
The insurance industry opposes these safeguards. UnitedHealthcare's Medicare CEO Tim Noel argues that restricting "utilization management tools would markedly deviate from Congress' intent." But as the NAIC emphasizes, meaningful transparency requires more than superficial disclosures - insurers must document and justify their AI systems' decision-making criteria, training data, and potential biases. Most critically, human clinicians with relevant expertise must maintain true decision-making authority, not just rubber-stamp algorithmic recommendations.
Recommendations for Action
The NAIC framework provides a roadmap for protecting patients while ensuring appropriate oversight of AI in healthcare claims. Key priorities for federal and state regulators:
Require comprehensive disclosure of AI systems' training data, decision criteria, and known limitations
Mandate documentation of physician recommendation overrides with clinical justification
Implement regular independent audits focused on denial patterns affecting marginalized communities
Establish clear accountability and substantial penalties when AI denials cause patient harm
Create expedited appeal processes for urgent care needs
Healthcare providers should:
Document all cases where AI denials conflict with clinical judgment
Track patient impacts from inappropriate denials, including worsened health outcomes
Report systematic discrimination in algorithmic denials
Support patient appeals with detailed clinical documentation
Share denial pattern data with regulators and policymakers
The solutions cannot rely solely on technological counter-measures. As the NAIC emphasizes, "The time to act is now."
Conclusion
The AI-driven denial of care represents more than a technological problem - it's a fundamental breach of the healthcare system's ethical foundations. By prioritizing algorithmic efficiency over human medical judgment, insurers have transformed life-saving care into a battlefield where profit algorithms determine patient survival.
Meaningful change requires a multi-pronged approach: robust regulatory oversight, technological accountability, and a recommitment to patient-centered care. We cannot allow artificial intelligence to become an instrument of systemic denial, transforming healthcare from a human right into an algorithmic privilege.
Patients, providers, and policymakers must unite to demand transparency, challenge discriminatory systems, and restore the primacy of human medical expertise. The stakes are too high to accept a future where lines of code determine who receives care and who is left behind. Our healthcare system must be rebuilt around a simple, non-negotiable principle: medical decisions should serve patients, not corporate balance sheets.
Biden’s Inaction Leaves Copay Assistance in Limbo
Inflated prescription drug costs in the United States continue to place a significant burden on people living with chronic conditions. Copay assistance programs, designed to help people afford their medications, have become essential. Yet recent policy decisions and industry practices have put these programs at risk, potentially jeopardizing access to necessary treatments.
The Biden Administration's recently proposed 2026 Notice of Benefit and Payment Parameters (NBPP) rule omits crucial regulations that patient advocates have long been demanding. This inaction allows insurers and pharmacy benefit managers (PBMs) to continue profiting from billions of dollars of drug manufacturer copay assistance intended for patients.
The State of Copay Assistance
Copay assistance programs, primarily offered by pharmaceutical manufacturers, provide financial support to help cover out-of-pocket costs for prescription medications. As health insurance plans increasingly shift costs to patients through higher deductibles and copayments, these programs have become crucial.
According to the latest data from The IQVIA Institute, manufacturer copay assistance offset patient costs by $23 billion in 2023, a $5 billion increase from the previous year. This figure represents 25% of what retail prescription costs would have been without such assistance. Over the past five years, copay assistance has totaled $84 billion, highlighting its importance in maintaining access to medications.
Despite the significance of copay assistance, copay accumulator and maximizer programs accounted for $4.8 billion of copay assistance in 2023—more than double the amount in 2019. Implemented primarily by pharmacy benefit managers (PBMs) and insurers, these programs prevent assistance from counting towards patients' deductibles and out-of-pocket maximums. This practice effectively nullifies the intended benefit of copay assistance, leaving people to face unexpected and often unaffordable costs later in the year.
Recent scrutiny of PBMs has brought attention to these practices. As discussed in our recent article on PBMs, the Federal Trade Commission (FTC) has filed a lawsuit against the largest PBMs for alleged anticompetitive practices that inflate drug costs and limit access to medications. These developments underscore concerns about how PBM practices, including the implementation of copay accumulator programs, impact medication affordability and access.
The impact on access is serious. IQVIA reports that in 2023, patients abandoned 98 million new therapy prescriptions at pharmacies, with abandonment rates rising as out-of-pocket costs increase. This trend highlights the critical role copay assistance plays in helping people not only initiate but also maintain their prescribed treatments.
Public opinion strongly supports action on this issue. A Kaiser Family Foundation survey found that 80% of adults believe prescription drug costs are unreasonable, with broad support for various policy proposals to lower drug costs. This sentiment reflects the public's recognition of the financial challenges faced in accessing necessary medications.
The Legal and Regulatory Landscape
The regulatory environment surrounding copay assistance programs has been in flux, with significant developments in recent years. On September 29, 2023, a federal court struck down a rule that allowed insurers to decide whether copay assistance would count towards patients' out-of-pocket maximums. This ruling reinstated the 2020 NBPP rule, which required insurers to count copay assistance towards patient cost-sharing, except for brand-name drugs with available generic equivalents.
Despite this, the federal government declared that it would not enforce the court's decision or the 2020 NBPP rule until new regulations are issued. This inaction has left patients facing continued uncertainty about the status of their copay assistance.
On January 16, 2024, the Biden Administration dropped its appeal of the court decision. While this action confirms that the 2020 NBPP rule will generally apply until new rules are issued, the lack of enforcement leaves plans and insurers in a gray area regarding their copay accumulator programs.
At the state level, there has been a growing movement to address copay accumulator programs. As of 2024, 21 states, the District of Columbia, and Puerto Rico have enacted laws addressing the use of these programs by insurers or PBMs. These laws generally require any payments made by or on behalf of the patient to be applied to their annual out-of-pocket cost-sharing requirement. While these state actions provide important protections, they do not cover all insurance plans, particularly those regulated at the federal level.
The 2026 Notice of Benefit and Payment Parameters Proposal
The proposed 2026 NBPP rule, released by the Centers for Medicare & Medicaid Services (CMS), has drawn criticism from patient advocacy groups for significant omissions related to copay assistance and essential health benefits (EHB).
Notably absent from the proposed rule are regulations clarifying whether copay assistance will count toward patient cost-sharing. This omission perpetuates uncertainty created by previous conflicting rules and court decisions, allowing insurers and PBMs to continue implementing copay accumulator programs that can leave people with unexpected and unaffordable out-of-pocket costs.
The proposal also fails to include a provision to ensure that all drugs covered by large group and self-funded plans are considered essential health benefits, despite previous indications that such a provision would be forthcoming. This failure to close the EHB loophole allows employers, in collaboration with PBMs and third-party vendors, to designate certain covered drugs as "non-essential," circumventing Affordable Care Act (ACA) cost-sharing limits designed to protect people from excessive expenses.
By exploiting this loophole, plan sponsors can collect copay assistance provided by manufacturers without applying it to beneficiaries' cost-sharing requirements. This practice effectively doubles the financial burden on patients: first, by accepting the copay assistance, and second, by requiring them to pay their full out-of-pocket costs as if no assistance had been provided.
Recent research by the HIV+Hepatitis Policy Institute has revealed that over 150 employers and insurers are taking advantage of the EHB loophole. This list includes:
Major companies such as Chevron, Citibank, Home Depot, Target, and United Airlines
Universities including Harvard, Yale, and New York University
Unions like the New York Teamsters and the Screen Actors Guild
States such as Connecticut and Delaware
Insurers, including several Blue Cross/Blue Shield plans
Patient advocacy groups have reacted strongly to these omissions. Carl Schmid, executive director of the HIV+Hepatitis Policy Institute, stated, "Every day these rules are delayed is another day that insurers and PBMs are pocketing billions of dollars meant for patients who are struggling to afford their drugs." This sentiment reflects the frustration of many who have long advocated for stronger protections.
The widespread exploitation of the EHB loophole underscores the urgent need for federal action to protect patients from these practices. The failure to address these critical issues in the 2026 NBPP proposed rule highlights a significant setback in efforts to improve medication affordability and access for people living with chronic conditions.
The Impact on Patients: Data and Experiences
The real-world impact of copay accumulator programs and the EHB loophole is reflected in both data and personal experiences. IQVIA reports that patient out-of-pocket costs reached $91 billion in 2023, an increase of $5 billion from the previous year. This rise in costs comes despite the $23 billion in copay assistance provided by manufacturers, highlighting the growing financial burden on patients.
Prescription abandonment is particularly concerning. Patients abandoned 98 million new therapy prescriptions at pharmacies in 2023, with abandonment rates increasing as out-of-pocket costs rise. More than half of new prescriptions for novel medicines go unfilled, and only 31% of patients remained on therapy for a year. These statistics highlight the direct link between cost and medication adherence.
People across the country are facing these challenges. For example, a mother whose daughter lives with cystic fibrosis shared her experience with a copay accumulator program. In early 2019, her family's out-of-pocket cost for her daughter's medication suddenly jumped from $30 to $3,500 per month when their insurance plan stopped applying copay assistance to their deductible. This unexpected change forced the family to put the cost on credit cards, creating significant financial strain and unnecessary medical debt.
Similarly, a person living with psoriasis faced steep increases in medication costs when their insurance company stopped counting copay assistance towards their deductible. The copay rose from $35 to $1,250 monthly, leaving them with only $26 from their disability payment after covering the copay.
These stories are not isolated incidents. People living with conditions such as HIV, hepatitis, multiple sclerosis, and hemophilia are facing similar challenges. The impact extends beyond financial stress, affecting medication adherence and, ultimately, health outcomes. For many, the choice becomes one between essential medications and other basic needs such as food and shelter—a decision no one should have to make.
Policy Recommendations and Advocacy Efforts
Patient advocacy groups are intensifying efforts for policy changes at both the federal and state levels. The All Copays Count Coalition, comprising over 80 organizations representing people living with serious and chronic illnesses, has been at the forefront of these efforts. In a letter to federal officials, the coalition urged for a revision of the cost-sharing rule to include clear protections ensuring that copayments made by or on behalf of a patient are counted towards their annual cost-sharing contributions. Specific recommendations include:
Maintaining the protections included in the 2020 Notice of Benefit and Payment Parameters.
Ensuring that copay assistance counts for medically appropriate medications, even when generic alternatives are available.
Limiting Health Savings Account-High Deductible Health Plan (HSA-HDHP) carve-outs to situations where using copay assistance would result in HSA ineligibility.
At the state level, advocacy efforts have led to the passage of laws restricting copay accumulator programs in 20 states, the District of Columbia, and Puerto Rico as of summer 2023. However, these state-level protections do not cover all insurance plans, particularly those regulated at the federal level, highlighting the need for comprehensive federal action.
Advocates are calling for:
Immediate enforcement of the 2020 NBPP rule, requiring insurers to count copay assistance towards patient cost-sharing in most cases.
Swift action to close the essential health benefits loophole for all plans, including large group and self-funded plans.
Increased oversight and regulation of PBM practices, particularly regarding copay accumulator and maximizer programs.
Passage of comprehensive federal legislation to protect those relying on copay assistance.
As Carl Schmid emphasized, "While they have gone on record that they will issue these rules, the clock is ticking and there isn't much time left." This reflects the growing frustration among patient advocates with the administration's delays in addressing these issues.
Policymakers must act swiftly to close the essential health benefits loophole and ensure that all copay assistance counts towards patients' out-of-pocket costs. Stakeholders across the healthcare ecosystem—from insurers and PBMs to pharmaceutical companies and patient advocacy groups—must collaborate to develop solutions that prioritize access and affordability. The health and well-being of millions depend on these critical policy changes.
Are Cancer Risks Higher for People Living with HIV/AIDS
For many years, scientists have been exploring the relationship between HIV/AIDS and various cancers. This complex connection stems from how the virus weakens the immune system, leaving folks more vulnerable. While there has been evidence of higher prevalence of certain cancers amongst people living with HIV/AIDS (PLWHA), the actual mechanisms have thus far remained unclear.
Recently, a team from Hospital 12 de Octubre (H12O) and the National Cancer Research Centre (CNIO) in Madrid, Spain found that Hepatitis B and C viral infections can cause multiple myeloma and its pathological precursors, monoclonal gammopathies. Additionally, they found that early detection of viral hepatitis and the use of antiretrovirals resulted in better health outcomes in general – taking care of the hepatitis and the monoclonal gammopathies/multiple myeloma at the same time.
This groundbreaking discovery comes shortly after the data was made available from the HIV/AIDS Cancer Match Study, which links United States’ HIV surveillance and cancer registry data from 2000 to 2019. Haas et al. examined this data and created a population-based linkage study which garnered the largest cohort to date for estimating anal cancer among PLWHA in the United States, with a cohort of 3,444 anal cancers diagnosed in patients with HIV. Of these 3,444 cases, 2,678 occurred in patients with a prior AIDS diagnosis.
Additionally, several cancers have been identified as AIDS-defining illnesses. The presence of these conditions indicates that the patient has reached the advanced stage of HIV infection known as AIDS. These include invasive cervical cancer, Kaposi’s sarcoma, and some iterations of lymphoma (e.g. diffuse large B-cell lymphoma and Burkitt or Burkitt-like lymphoma). A 2021 meta-analysis of twenty-four studies found that women with HIV are six times more likely to have cervical cancer than their counterparts without HIV. This is likely due to the inability to clear human papillomavirus (HPV) infection, which can cause cervical cell changes if left untreated in some cases. According to Lymphoma Action, diffuse large B-cell lymphoma is around fifteen times more common in PLWHA, while PLWHA are around 30 times more likely to develop Burkitt lymphoma.
As is often the case when living with HIV/AIDS and co-occurring conditions, early detection, open and frequent communication with healthcare providers, and HIV/AIDS treatment regimen adherence can make a significant difference in the duration and intensity of these conditions, so patients should be encouraged to be vigilant self-advocates when it comes to their health and wellness, and, when needed, identify resources among caregivers and community who might be able to assist in care advocacy.
Eddie Hamilton (left), Bill Arnold (right)
CANN would like to recognize the fierce advocacy of our colleague, friend, and former board member, Edward “Eddie” Hamilton (pictured). Eddie served on CANN’s Board of Directors from 2014 to 2022 and was the Founder and Executive Director of the ADAP Educational Initiative, which assisted clients enrolled under the AIDS Drug Assistance Program (ADAP). In 2012, Eddie was honored as the ADAP Champion of the Year by ADAP Advocacy for fighting the Ohio Department of Health’s attempts to implement medical eligibility criteria to qualify for ADAP services. Eddie passed away on July 12, 2022, having had cancer twice. CANN continues to honor Eddie’s legacy as well as that of the late Bill Arnold (pictured right), who served as CANN’s founding President & CEO, by championing patient-centric action toward health equity and access.
Profit Over Patients: Challenging the Understaffing Crisis in Healthcare
On December 31, 2023 former U.S. Representative Eddie Bernice Johnson suffered and died in a completely preventable yet entirely foreseeable tragedy. Rep. Johnson, a dedicated nurse and a fervent advocate for equitable healthcare, succumbed to an infection contracted in a rehabilitation facility, a direct consequence of medical neglect. This incident is a glaring example of the systemic issues plaguing our healthcare institutions, where intentional understaffing and profit-driven motives often come at the expense of patient care and staff well-being. Her experience tragically highlights the broader systemic issues in healthcare, including rampant understaffing and the consequences of healthcare system consolidation.
The Tragic Circumstances of Rep. Johnson's Passing
According to a Texas Tribune report, Rep. Johnson died a “terrible, painful death” from an infection caused by negligence at her Dallas recovery facility following back surgery. The infection was a result of being left to lie in her own feces and urine for roughly an hour while she repeatedly called for help that didn’t come. The facility reportedly told family that all staff were unavailable as she called for help due to being in a training. Her son, Kirk Johnson, minced no words as he stated, "She was screaming out in pain, asking for help. If she had gotten the proper care, she would be here today.”
The family notified Baylor Scott & White Health System and Baylor Scott & White Institute for Rehabilitation of their intention to sue on the grounds of medical negligence. The lawsuit, if not settled, will highlight the deadly consequences of inadequate patient care in healthcare facilities. This legal battle is complicated by Texas law, which limits medical malpractice lawsuit awards to $250,000. Such legislative decisions, influenced by powerful hospital lobbies, not only restrict legal recourse for patients but also reflect deeper systemic issues in healthcare governance where institutional profits often overshadow patient rights.
The limitation on medical malpractice awards in Texas exemplifies a troubling trend in healthcare legislation. These laws, as detailed in a Miller & Zois report, often protect healthcare institutions at the expense of patient health and safety while significantly limiting patients' ability to seek fair compensation for medical negligence.
This legislative backdrop, coupled with intentional understaffing in healthcare facilities, creates a perilous situation where patient rights are limited and institutions are insulated from liability when their cost cutting measures cost lives. Maximizing profit and administrative and shareholder value by understaffing care facilities heightens the risk of medical errors, burns out staff, and creates unsafe working conditions. Yet, when these cost-cutting measures lead to harm, patients find their legal recourse severely restricted by malpractice caps while hospital staff burns out and are exposed to greater occupational hazards. The only ones not on the losing end are the hospitals and their executives.
Staffing Shortages or Healthcare Profiteering?
Across the country, as healthcare corporations report burgeoning profits, the reality within their healthcare facilities tells a story of compromised care and strained resources. Let’s take Hospital Corporation of America (HCA), the largest hospital system in the country, as an example. As reported in The Guardian, a study by the Service Employees International Union (SEIU) highlights the disparities, revealing that staffing ratios at HCA Hospitals in 2020 were alarmingly 30% lower than national averages. Despite $7 billion in profits and $8 billion allocated to stock buybacks and paying out nearly $5 billion in dividends to shareholders, the investment in patient care, particularly in terms of staffing, remains inadequate.
The prevailing narrative of a nursing shortage in the United States is rigorously challenged by facts and voices from within the healthcare sector. National Nurses United (NNU) asserts that the core issue is not a lack of nurses but rather the widespread unwillingness of nurses to work under unsafe conditions. This perspective contradicts the healthcare industry's narrative and points to systemic issues in workforce management and underinvestment in medical staffing by hospital executives.
The intentional understaffing by healthcare facilities, as seen in cases like HCA Hospitals, is often driven by financial motivations. By keeping staffing levels low, these facilities aim to maximize profits, often at the expense of both patient care and staff well-being. This approach has led to a situation where the healthcare workforce is being pushed to its limits, leading to high turnover rates and a growing reluctance among nurses to work in such conditions.
The narrative of worker shortages is further complicated by the trend of healthcare system consolidation, which significantly reshapes healthcare markets, often at the expense of patient care and staff well-being. In May of 2023 The RAND Corporation gave testimony to the U.S. House of Representatives Committee on Ways and Means, Subcommittee on Health which underscored that consolidation frequently leads to higher healthcare costs without corresponding improvements in quality. Characterized by mergers and acquisitions across markets, this trend typically results in reduced competition, higher prices, and a focus on revenue generation over patient-centric values. Moreover, when private equity is involved, as highlighted by The British Medical Journal (BMJ), it often exacerbates patient harm.
The Human Cost of Cost-Cutting
Impact on Healthcare Workers: Nurses and other healthcare staff, the backbone of patient care, are stretched to their limits. A study by the University of Pennsylvania highlights the high levels of nurse burnout, a direct consequence of inadequate staffing. The study surveyed over 70,000 nurses and found that the chronic stress caused by high nurse-to-patient ratios significantly impacts their mental and physical health. The turnover rate in nursing, as reported by STAT News, is a testament to the unsustainable working conditions, with many nurses leaving the profession or seeking less demanding roles.
Patient Safety and Care Quality: The impact of understaffing on patients is equally alarming. According to the National Center for Biotechnology Information (NCBI), inadequate staffing in nursing homes is linked to increased incidents of falls, bedsores, and a general decline in the quality of care. This neglect is not limited to nursing homes; hospitals across the nation face similar challenges. As in the case of Rep. Johnson, patients often experience delayed care, unmet basic needs, and an increased risk of medical errors due to the high workload on understaffed healthcare workers.
The understaffing crisis extends beyond individual facilities. As National Nurses United points out, the issue is systemic and has become an industry standard practice. These ethically dubious practices have far-reaching consequences, eroding the sustainability of the healthcare system and diminishing public trust in its ability to provide competent and compassionate care.
Upholding Ethical Standards in Healthcare
The ethical implications of understaffing and system consolidation are profound. It's not merely a matter of operational efficiency; at its core, it's about honoring a fundamental commitment to patient care and worker dignity. The primary ethical concern in healthcare should be the obligation to provide safe, effective, and compassionate patient care, an obligation that is often directly undermined by profit-driven decisions.
The direct consequences of understaffing and consolidation, such as compromised patient safety, increased medical errors, and a decline in the quality of care, represent a breach of the ethical duty healthcare providers owe to their patients. When financial priorities overshadow patient needs, the very essence of healthcare's moral foundation is shaken. This shift not only impacts patient outcomes but also erodes public trust in healthcare systems.
The alarming levels of burnout, stress, and turnover among healthcare workers, particularly nurses, reflect a work environment that neglects their physical, emotional, and professional well-being. This neglect raises serious ethical concerns about the healthcare industry's commitment to its workforce. When staff well-being is compromised for operational efficiency or financial gain, the entire healthcare system suffers, leading to a demoralized workforce and diminished patient care.
The healthcare industry faces a critical ethical dilemma: balancing financial responsibilities with the imperative of humanistic care. While healthcare facilities have fiscal duties to their stakeholders, these must never be allowed to eclipse their ethical obligation to prioritize high-quality patient care and foster a safe and supportive work environment. The pursuit of profit must be balanced with the moral imperative to care for both patients and healthcare workers humanely. This balance is essential not only for the integrity of healthcare providers but also for the long-term sustainability of the healthcare system as a whole.
Addressing these ethical challenges is not just a moral imperative but a crucial step towards systemic reform for a more humane and effective healthcare system and, frankly, reducing costs to patients by way sufficient retention of nursing talent - reduced turn over means reduced labor costs which then translates to reduced insurance billing and less medical debt.
Concrete Steps Towards Reform
The reality of understaffing and the challenges posed by healthcare system consolidation in our healthcare system demand immediate and decisive action. We must engage in targeted advocacy and policy reform. Here are specific actions that individuals and organizations can undertake to drive meaningful change:
Contact Legislators: Advocate for federal and state legislation that mandates safe staffing ratios in healthcare facilities, addresses the challenges of healthcare consolidation and transparency, and holds hospitals accountable for malpractice. This includes challenging laws that limit malpractice awards, as these can protect healthcare institutions at the expense of patient rights.
Support Nursing Unions: Participate in advocacy campaigns of unions like National Nurses United, supporting their efforts for better working conditions and fair staffing levels. These unions play a crucial role in voicing the concerns of healthcare workers and advocating for their rights.
Engage with Healthcare Boards: Advocate for ethical staffing practices and policies that prioritize patient care over profit in healthcare organization board meetings. It's essential to influence decision-makers at the highest level to bring about systemic changes.
Advocate Against Unchecked Consolidation: Support policies that scrutinize healthcare mergers and acquisitions, as highlighted by the National Conference of State Legislatures (NCSL), to ensure they prioritize patient care and staff welfare. This includes backing state and federal initiatives to enhance oversight on healthcare mergers and acquisitions.
We must shift the focus from profit margins to the pillars of empathy, compassion, and quality care. It's time to honor the legacy of advocates like Rep. Eddie Bernice Johnson and ensure that our healthcare system upholds its fundamental commitment to patient care and worker dignity. Implementing these actions can lead to a more empathetic, compassionate healthcare environment, where patient care and staff welfare are prioritized, paving the way for a sustainable and trustworthy healthcare system.
Upper-Payment Limits; Drug "Affordability" Boards Risk Medication Access
The opinion piece, authored by Jen Laws, CANN’s President & CEO, originally published in the September 2, 2023, print edition of the Denver Post. CANN will be hosting a free “PDAB 101” webinar for patients, advocates, and all public health stakeholders on November 1, 2023. Pre-registration is required. Register by clicking here.
To successfully combat the HIV epidemic and defeat other chronic conditions, patients must have uninterrupted access to the most effective medicines recommended by their doctors. As efforts to ensure patients can access their medicines are being defined in the public sphere, many state legislatures continue to advance policies and proposals focused on addressing patient affordability challenges.
However, many such actions fail to address high out-of-pocket costs and instead focus on lowering costs for other stakeholders within the health care system, like lowering costs and increasing profits for health insurers neglecting the patients they were intended to protect.
In Colorado and several other states across the country, lawmakers have empowered Prescription Drug Affordability Boards (PDABs) to address the rising costs that patients pay for prescription medicines. PDABs have the authority to select and review drug list prices and can recommend policies for drugs deemed "unaffordable." These list prices aren't something patients generally pay, rather we pay co-pays or are able to manage costs with patient assistance programs.
Despite this, one such policy being considered by the Colorado PDAB and similar boards in other states is an upper-payment limit (UPL). A UPL is a payment limit or ceiling that applies to all purchases and payments for certain high-cost drugs and does not necessarily translate into a "cost limit" for patients.
When UPLs are set, reimbursement rates are lowered for hospitals or clinics giving them less incentive to purchase specific drugs even though it may be the most effective medication to help a patient manage a chronic condition. When reimbursement rates are lowered through a UPL, it can also lead to barriers to biopharmaceutical companies investing in and supplying new innovative medicines to health facilities, making it difficult for doctors to prescribe treatments they think are best suited for their patients. While well intentioned, patients often bear the brunt of the challenges with such policies.
The impacts of the UPL process are only compounded when we consider the potential impact on the 340B Drug Pricing Program, a federal safety-net program that helps health facilities serve low-income and uninsured patients by offering them discounted drugs. Under the program, qualified clinics and other covered entities buy treatments at a discount to help treat vulnerable patients and get to keep the difference between the reimbursement rate and the discounted price leveraging those dollars to provide needy patients with medications and care they might not otherwise be able to afford.
Under a UPL, health facilities such as hospitals or clinics will receive lower reimbursements for prescribed treatments and therefore generate fewer dollars to support patients and the care we need to live and thrive. If the PDAB sets restrictive UPLs for drugs for chronic conditions like HIV, health facilities and the health professionals tasked with providing care will be faced with the decision to potentially stop prescribing these medicines and face having to cut support services that patients have come to rely on.
At a recent meeting of Colorado PDAB stakeholders, following the board's unanimous approval of the list of drugs eligible for an affordability review process, I voiced concerns about the approach to determining the value of lifesaving treatments for patients living with or at risk for HIV, hepatitis C (HCV), and other complex conditions. My concerns have only grown since, most recently, the state PDAB selected five drugs to undergo a formal affordability review including a treatment for HIV.
Many patients and other stakeholders have raised alarm to other drugs that are now subject to review to treat complex conditions such as psoriasis, arthritis, psoriatic arthritis, and cystic fibrosis. The implications of the Colorado drug "affordability" board's recent actions on patient access are grave and set a dangerous precedent. Ten states including Colorado have already established PDABs, and many others are following suit.
Those support services and continuity of care are critical to empower communities and improve the quality of life for people living with and managing conditions like HIV and hepatitis C. Despite the PDAB being "sold" to the public as a measure to improve patient experiences and access to care, the current model fails to prioritize patients at all.
Colorado is home to more than 13,000 people living with HIV and has been at the forefront of combating the disease. This year, state lawmakers advanced model legislation that protects patients' access to HIV prevention medication. However, the recent actions from the drug "affordability" board and short-sighted policies like the UPL process or mandatory generic switching could derail progress toward ending the HIV epidemic.
Price controls are, and will continue to be, a short-term, short-sighted "fix" with long-term consequences for patients living with chronic conditions. Policy efforts to address affordability must prioritize patient access and the ability for doctors to prescribe effective treatments. Colorado's PDAB, as it currently stands, falls short of that.
Unveiling Disparities: OIG Report on HIV Care in Medicaid
A recent report by the U.S. Department of Health & Human Services’ (HHS) Office of Inspector General (OIG) unveiled a startling revelation: one in four Medicaid enrollees with HIV may not have received critical services in 2021. At a time when healthcare systems worldwide were grappling with the COVID-19 pandemic, these findings highlight the compounded struggles faced by People Living with HIV (PLWH) in accessing essential care.
Beyond safeguarding the health of PLWH, viral suppression plays a pivotal role in curbing HIV transmission, a critical metric in the public health effort to end HIV. In alignment with HHS guidelines, achieving consistent viral suppression necessitates three foundational elements: routine medical consultations, ongoing viral load assessments, and unwavering commitment to ART regimens.
Unveiling the Care Gaps
The OIG's analysis reveals pressing challenges within our system:
Out of the 265,493 Medicaid enrollees diagnosed with HIV, a startling 27% were missing evidence of receiving at least one of the pivotal services last year.
10% were missing evidence of medical visits, and 11% were missing evidence of an ART prescription, raising concerns around disease progression and higher incidence of AIDS diagnosis, increased risk of transmitting the virus, and developing antiviral resistance.
The most pronounced gap? Viral load tests. A staggering 23% of enrollees lacked evidence of even a single viral load test in 2021. This absence not only impedes clinicians from making informed decisions but also hampers our collective ability to monitor and respond to the evolving nature of the HIV epidemic.
Perhaps most concerning is the fact that more than 11,000 of these enrollees didn't have evidence of availing any of the three critical services. This is not just a statistic; it's a reflection of real individuals, facing amplified health risks due to system inadequacies.
Jen Laws, CANN's President and CEO, poignantly remarked, "Medicaid represents the greatest public program coverage of PLWH. It also represents the greatest public program coverage of people at risk of acquiring HIV. Medicaid compliance and efficacy is critical to Ending the HIV Epidemic and this report has identified gaps where states have failed to meet their obligations to Medicaid beneficiaries and where CMS has failed to ensure compliance. We must do better if we are going to reach our public health goals."
The report underscores stark disparities in care access between Medicaid-only and dual-eligible enrollees (those with both Medicaid and Medicare). Specifically, Medicaid-only enrollees were three times more likely to lack evidence of any of the three critical services compared to dual-eligible enrollees, with 6% of Medicaid-only enrollees missing out, as opposed to just 2% of the dual-eligible group. Such disparities might stem from various factors, including Medicare's higher fee-for-service rates and the observed long-standing adherence patterns among older adults who have had HIV for prolonged periods.
State-wide Disparities: A Complex Landscape
The disparities in HIV care access across states offer both a grim reality check and a clarion call for systemic reform. Drawing from the OIG report, certain states, notably Arizona, Arkansas, the District of Columbia, and Utah, have alarmingly high proportions of Medicaid-only enrollees without evidence of at least one of the three critical services. For instance, Utah stands out with a staggering 87% of such enrollees missing out on essential care. These aren't just numbers; they represent real individuals grappling with a system that's failing them.
Conversely, some states demonstrate better compliance and efficacy in delivering HIV care, which suggests potential models or strategies that could be emulated across the board. However, the stark variability across states points to the undeniable influence of state-specific policies, the quality of local HIV care infrastructures, and broader challenges associated with healthcare access.
State agencies clearly have much work ahead. These disparities not only indicate potential inefficiencies or gaps in policy implementation but also suggest a pressing need for introspection and reform at the state level. Coupled with challenges in the broader Medicaid system, there's a compelling case for a comprehensive overhaul.
The onus is on both state agencies and the Centers for Medicare & Medicaid Services (CMS) to rise to the occasion. The disparities, as evidenced in the report, necessitate a deep dive to understand the underlying causes. Is it a matter of policy misalignment, funding constraints, administrative challenges, or a combination thereof?
For instance, the significant variation in care access among dual-eligible enrollees, ranging from 9% to 53% across states, speaks volumes about the discrepancies in policy implementation and oversight. It's vital to pinpoint these issues, develop tailored interventions, and ensure that every Medicaid enrollee, irrespective of their state, receives the essential services they need.
The Pandemic's Shadow
The COVID-19 pandemic exerted immense pressure on healthcare systems, with profound implications for HIV care. The OIG report highlights the challenges faced by Medicaid enrollees with HIV during the COVID-19 pandemic. A significant finding from the report is the notable deficiency in viral load tests among these enrollees. This underscores the need for healthcare systems to effectively manage and prioritize essential services, even amidst broader healthcare crises. The report serves as a reminder of the importance of consistent and uninterrupted care for conditions like HIV, even when healthcare systems face external pressures.
Steering Towards a Brighter Future
Addressing the glaring disparities laid out in the OIG report goes beyond mere policy recalibration; it challenges our collective resolve to uphold equitable healthcare. In the shadow of the pandemic's aftermath, it's imperative that essential services for PLWH are not just nominally available but are genuinely accessible.
The OIG report's findings don't merely spotlight discrepancies; they highlight systemic lapses. States have fallen short in fulfilling their obligations to Medicaid beneficiaries, and the CMS has not adequately ensured compliance.
To translate this call to action into meaningful change, it's essential to:
Strengthen state-level accountability frameworks to ensure Medicaid obligations are met comprehensively, particularly among states utilizing Managed Care Organizations – ensuring those contracted for-profit insurance companies are meeting their contractual obligations to states. The tools for accountability already exist within these contracts, they merely must be used.
Bolster CMS oversight mechanisms, driving proactive interventions that hold states to account and rectify compliance lapses.
Engage in continuous dialogue with stakeholders, including healthcare providers and beneficiaries, to identify and remedy bottlenecks in care access.
The findings from the OIG report serve as a stark reminder of the work ahead. As Jen Laws aptly states, "We must do better." HIV advocates should consider assessing our engagement with their state Medicaid programs and look for opportunities to act, akin to our engagement Ryan White programs. With concerted efforts, policy reforms, and collective commitment, we can bridge the gaps and ensure comprehensive care for all PLWH.
Prescription Drug Advisory Boards: Who is Impacted and How to get Involved
The prescription drug advisory board (PDAB) train keeps chugging along. Presently, there are nine (9) states that had, have or are in the process of enacting PDAB legislation: Washington, Oregon, Colorado, Michigan, Minnesota, New Jersey, New Hampshire, Maryland, and Maine. Ohio, it would seem, has abandoned their PDAB efforts. Their geographical variance reflects the diversity of their structures. Some of the boards have five members, and some have seven. While all are appointed by the governor, they differ regarding which departments they are associated with. For example, Colorado’s is associated with the Division of Insurance, and Oregon’s is associated with the Department of Consumer and Business Services.
The assortment of structure does not stop at department association. The number of drugs to be selected annually for review also varies, such as Colorado with five and Oregon with nine. Even the number of advisory council members lacks consistency. The New Jersey DPAB advisory council has twenty-seven (27) members, while Colorado’s has fifteen (15). Inconsistency in structure means inconsistency in operations. Thus, the help or harm patients ultimately receive will vary drastically from state to state. The most important differences are the powers bestowed upon the various DPABs. In addition to shaping many policy recommendations, five (5) currently have the ability to enact binding upper payment limit (UPL) settings: Washington, Oregon, Colorado, Maryland, and Minnesota.
An upper payment limit sets a maximum for all purchases and payments for expensive drugs. By setting UPLs for high-cost medications, improved ability to finance treatment equals greater access to high-cost medicines. A UPL sets a ceiling on what a payor may reimburse for a drug, including public health plans, like Medicaid.
Patients, advocates, caregivers, and providers are concerned about PDABs because the outcomes of theory versus practice can have dire consequences. Theoretically, PDABs should reduce what patients spend out of pocket for medications and lower government prescription drug expenditures. However, the varied ways different PDABs are set to operate could jeopardize goals. Focusing on lowering reimbursement rates could affect the funds used as a lifeline by organizations benefiting from the 340B pricing program even while not meaningfully reducing patient out-of-pocket costs. If reimbursement limits are set too low, those entities will have drastic reductions in the funding they use for services for the vulnerable populations they serve. UPLs could ultimately increase patients' financial burden if payers increase cost-sharing and change formulary tiers to offset profit loss from pricing changes or institute utilization management practices like step-therapy or prior authorization. Increasing patient administrative burden necessarily decreases access to medication. When patients are made to spend more time arguing for the medication they and their provider have determined to be the best suited for them, rather than simply being able to access the medication, the more likely patients are to have to miss work to fight for the medication they need or make multiple pharmacy trips – or suffer the health and financial consequences of having to “fail” a different medication first. PDAB changes could affect provider reimbursement, which could be lowered with pervasive pricing changes. Decreased provider reimbursement could result in additional costs being passed onto patients or, in the situation of 340B, safety-net providers, reduce available funding for support services patients have come to rely upon.
The divergent factors that different PDABs use for decision-making are of concern as well. It is not enough to just look at the list price of drugs and the number of people using them. For example, some worthwhile criteria for consideration of affordability challenges codified in Oregon’s PDAB legislation are: “Whether the prescription drug has led to health inequities in communities of color… The impact on patient access to the drug considering standard prescription drug benefit designs in health insurance plans offered…The relative financial impacts to health, medical or social services costs as can be quantified and compared to the costs of existing therapeutic alternatives…”. But few of these PDABs consider payer-related issues like limited in-network pharmacies, discriminatory reimbursement, patient steering mechanisms, or frequency of utilization management as hindrances to patients getting our medications.
Effectively seeking and considering input from patients, caregivers, and frontline healthcare providers is also of concern. The legislation of various DPABs specifies the conflicts of interest that board members cannot have and must disclose. Some even have appointed alternates to allow board members to recuse themselves from making decisions on drugs with which they have financial and ethical conflicts. However, most of the advisory boards are providers, government, and otherwise industry-related. The board members are even required to have advanced degrees and experience in health economics, administration, and more. The majority of the discourse is not weighted towards the patient and our advocates. Few, if any, specific active outreach measures when it comes to seeking patient input. For example, the Ryan White HIV/AIDS Program requires patient and community engagement outlets in planning activities. But no PDAB legislation, to our knowledge, requires PDABs to engage with these established patient-oriented consortia. We know well in HIV that expecting already burdened patients often struggle to meet limit engagement opportunities from government boards – we know the very best practices are going to patients, rather than expecting patients to come to these boards. Beyond these limited engagement opportunities and failure to reach out to spaces where patients are already engaged, some states have exceptionally short periods in which to gather these inputs.
However, depending on the individual state’s DPAB structure, there is an opportunity for patients, caregivers, and organizations to give input through public comment periods and particular meetings aimed at stakeholder engagement. For states considering PDAB legislation, like Michigan, patients can and should engage in the legislative process. One place to keep abreast of different state’s PDAB activities is the Community Access National Network’s PDAB microsite. The microsite has an interactive map where you can access various states’ PDAB sites as they are created. States with fully formed PDABs have sites that display their scheduled meetings, previous decisions made, agendas for future sessions, and, most notably, details of the process for the public to provide input. Most of the meetings are open to the public, with the public invited to provide oral public comment or to submit written comments. Attending meetings and speaking directly to the boards is a way to have board members and others hear directly from those who will be affected by their decisions. Written public comment is also essential, especially from community patient advocacy organizations. Some DPABS also provide access to virtual meetings where stakeholders can provide feedback and input.
Medicare has six protected drug classes: anticonvulsants, antidepressants, antineoplastics, antipsychotics, antiretrovirals, and immunosuppressants. This means that Medicare Part D formularies must include them but that protection exists because we know how important these medications are. Antiretrovirals and oncology medications are a part of that list because adversely affecting the mechanisms of access to those drug classes is life-threatening to those who need them. It is imperative that continued scrutiny be placed upon DPABs to ensure that their benefits are patient-focused, like reducing administrative burden and barriers to care, rather than a mask that ultimately benefits payers by increasing their profits.
Prescription Drug Advisory Boards: What They Are and Why They Matter to Patients
It’s no secret that the high cost of healthcare is a significant concern for most Americans. The total national health expenditure in 2021 increased by 2.7% from the previous year to 4.3 trillion dollars which was 18.3% of the gross domestic product. The federal government held the majority of the spending burden at 34%, with individual households a close second at 27%. A cornerstone component of medical treatment is the access to prescription drugs. In 2019 in the U.S., the government and private insurers spent twice as much on prescription drugs as in other comparatively wealthy countries. Despite catchy phrases that poll well, and “simple” solutions by politicians that promise to fix the problem—such as Prescription Drug Advisory Boards (also known as Drug Pricing Advisory Boards)—it is mindful to remember one thing: if it sounds good to be true, then it probably isn’t true.
CANN PDAB infographic: What are they and why do they matter? (https://tiicann.org/dpab-project.html)
While list prices of prescription drugs continue to increase, medication costs do not represent the largest share of healthcare costs or the largest growth in healthcare costs in the United States. The cost burden on patients is so untenable for many that some have to decide between paying for medications, food, or mortgages. However, due to a number of incentives and the role of loosely regulated pharmacy benefit managers (PBMs), there is little direct relationship between drug list prices and patient cost burdens. This fact is only just now being appreciated by lawmakers but is not currently reflected in our healthcare funding schemes. As such, the discourse surrounding lowering cost is a consistently turbulent sea navigated by diverse public and private parties, with the language around drug pricing assuming efforts to curb costs relate to patient costs and access – but not explicitly saying so (and for good reason). Some proposals are government related, such as federal drug pricing proposals. Recent developments are state-level focused closer to home. One such development is the Prescription Drug Advisory Boards, or PDABs.
PDABs are part of state divisions of insurance. Drug pricing efforts, in the general sense, could be a good thing. PDABs are being marketed to the public as a better means to make drugs more affordable for patients. However, the details of the implementation of developing PDABs are wherein lies significant challenges. Overall, the boards focus specifically on the prices of the drugs. However, the focus on pricing is mainly related to what governments, insurance companies, hospitals, and pharmacies are paying for the medications. This purview and the monitored metrics associated with PDABs do not necessarily translate into the actual costs patients pay at the pharmacy counter.
Because these designs are singularly focused on the “cost” to payors, current proposals and initiatives benefit both public and private payors at the expense of the patient access and the provider-patient relationship. It is unacceptable for any planned PDAB activity to disrupt the patient-provider relationship. Community Access National Network (CANN) has consistently opposed any policy initiative that might increase administrative barriers and patient burdens. Two examples are step-therapy and prior authorization. Activities such as these are considered what is known as utilization management. Utilization management helps lower prescription drug spending for public and private payors but creates additional costs for patients financially and logistically, affecting their continuity of care, amounting to a cost burden shift, not a meaningful increase of access to affordable, high-quality care and treatment for patients.
Additionally, the narrow specific focus on the list prices of drugs overlooks essential issues. Lowering the list price for medications can, for example, harm organizations that depend on revenues from the 340B Drug Pricing Program. The 340B program allows safety net clinics and organizations to purchase prescription drugs from manufacturers at a discounted price while being reimbursed by insurance carriers at a non-discounted cost. The surplus enables these entities to provide many services that the low-income populations they serve depend on. This is especially vital to low-income people living with HIV that do not have the means to afford all of their healthcare needs.
It is imperative that PDABs receive input directly from patients and caregivers as well. PDABs are aggregating a large amount of data. However, more of that data needs to include considerations of the patient experience. For example, drug rebate reductions can impact care and support services, such as transportation assistance or mental health services at federally qualified health centers (FQHCs). Moreover, there needs to be an examination of the actual pass-through savings to patients. Most importantly, PDABs need to explore how pricing decisions affect patient access. A lower drug list price is not beneficial to patients if it creates or increases administrative burdens or increases costs for patients in other ways outside of paying for the cost of medication.
Most policymakers do not always have robust experience in understanding the nuances of dealing with public health programs, clinics, and populations. This is especially true regarding the marginalized community of people living with or at risk for acquiring HIV, those affected by Hepatitis C, or people who use drugs. PDABS must be held accountable for acquiring anecdotal qualitative and quantitative data regarding patient experience, accessibility, and affordability while developing recommendations related to drug pricing. As it stands, of the states that have implemented a PDAB, none have statutorily mandated metrics monitoring patient experience and access.
Patients, caregivers, and advocates with direct experience and greater understanding of the policy landscape around healthcare access play a vital role in helping to shape legislation and informing proper implementation of programs to meet the goals those programs were “sold” on. If monitored metrics do not consider or reflect patient experiences, then the program is simply not about increasing access for patients.
PDABs, fortunately, do have numerous opportunities for patients, caregivers, advocates, and providers to become involved and to elevate patient priorities over that of other stakeholders. Getting involved and staying involved with a state’s PDAB work is critically necessary to ensure any final work or regulation is patient-focused.
CANN will be present and offering feedback at various PDAB meetings in affected states. The next meeting CANN will be attending is virtual for the state of Colorado, on July 13th at 10am Mountain time. You can register here and participate in ensuring any action taken reflects patient needs.
An Expression of Support for Basic Human Decency
Earlier this week, ADAP Advocacy Association and Community Access National Network (CANN) issued a joint statement announcing an embargo of each respective organization’s patient advocacy and education activities within the state of Florida. Both organizations also cited a need to protect advocates and patients from outside of the state from the very real dangers associated with traveling to the state, while also emphasizing that both organizations will continue to support local advocates in the state as they work to create positive public policy changes for Floridians living with HIV. The decision to adjoin both the ADAP Advocacy Association and the Community Access National Network to the previously issued formal travel advisory by the NAACP wasn’t taken lightly because maintaining strong ties to the community is important in generating effective advocacy. The move wasn’t a political statement either, but rather an expression of support for basic human decency.
The announcement comes after the state’s governor, Ron DeSantis, signed into law a series of bills targeted toward harming Black, Brown, LGBTQ+, and immigrant people. The transgender community was probably singled out more viciously than any of the marginalized communities throughout this hate-inspired Florida Legislative Session. Make no mistake about it why this effort to enflame a “culture war” is an issue of organizational values and something quite personal to both of us. The non-trans guy here taking issue with the fact that the trans guy here now cannot take “a leak” without fear of being charged with a felony has nothing to do with politics and everything to do with basic human rights.
The fact is we both previously lived in the state for many years – it’s where we started our HIV policy work, even before we knew one another. It is where we met over a decade ago. Upon reflection, we still can regularly be found discussing mutual friends from Florida, those still living and those who have passed on, in different phases of their lives.
From recalling Bishop S.F. Makalani-MaHee's testimony to the Florida Legislature in 2016, against a bathroom bill (which failed that year), to his death on Transgender Day of Remembrance in 2017, part of this internal discussion was a reflection on the deep history he had with advocates serving both the Transgender and HIV communities of the state. What we’re witnessing right now in Florida is challenging for us, personally and professionally, but state-sponsored discrimination, hate, and stigma drew a line that cannot be ignored.
In 2017, Human Rights Watch published an important report, Living At Risk: Transgender Women, HIV, and Human Rights in South Florida and the very same year ADAP Advocacy Association published it’s issue paper, Transgender Health: Improving Access to Care Among Transgender Men & Women Living with HIV/AIDS Under the AIDS Drug Assistance Program. Both of us worked on the ADAP project, and it was important for a transgender advocate (Jen) with lived experience to lead in writing model policies meant to serve Transgender People Living with HIV. The decision to issue a travel advisory in Florida for people living with HIV is rooted in disparities and areas of improvement emphasized in those two reports.
Much of our hearts belong to Florida for the dedication and innovation the people of this state can and do offer, despite every unnecessary public policy challenge they face. People like Mick Sullivan and Donna Sabatino (formerly with Tibotec Therapeutics), Connie Reese and her amazing work with Simply Amazing You Are (SAYA) in Miami-Dade County, Riley Johnson promoting trans equality in accessing medical care via RAD Remedy, Michael Ruppal’s leadership with The AIDS Institute, and the late Tiffany Marrero, who served to voice the experiences of vertical transmission patients and Black Women and only recently left us. Heck, Trelvis Randolph and Maria Mejia both reside in South Florida, and they serve on CANN’s board of directors. These folks not only are colleagues, but they are friends and expressing concern over traveling to a place once call “home” saddens us.
But some things are larger than us. Recognizing the inherent roots of racism, which has prompted the NAACP to issue a travel advisory, our joint statement read, in part:
The state of Florida's moves to harm Transgender people, Black and Brown communities, and immigrant families undermines the exceptional work the state's Health Department has done in the last several years and only serves to further existing health disparities affecting these communities, particularly as it relates to HIV. For example, according to Florida's own data, while Black and Hispanic/Latino communities make up about 15.6% and 26.7% of the state's population, respectively, these same communities represent 37.7% and 39.6% of HIV diagnoses. Put another way, in Florida, while white people experience a rate of HIV diagnoses of 8.5 per 100,000 people, that rate among Black communities is 51.8 and for Hispanic/Latino communities it's 31.7.
Similarly, Florida has, in years past, made extraordinary strides in ensuring transgender people can access HIV related care, specifically by integrating best practices and guidance from the Health Resources and Services Administration (HRSA) on integrating gender affirming care into HIV care provision. Indeed, as a result of these moves, transgender women represent some of the greatest successes in linkage to care, retention in care, and viral load suppression of any demographic in the state. Recently signed bills prohibiting state contracted clinics from providing gender affirming care will have a dramatic affect in reversing these long sought after wins.
Make no mistake, we are frustrated with an apparent lack of involvement from the federal agency charged with implementing the Ryan White HIV/AIDS Program. Because Ryan White program dollars are passed through the state and then contracted with counties, local areas, or directly with a provider, and because other health initiatives of the state are also part of how providers in Florida acquire funding to provide public health services, they may be prohibited from providing gender affirming care at all - regardless of where those dollars originate (state or Federal).
It is incumbent upon HRSA to provide guidance beyond ‘allowable’ uses and inform that state it has contractual, fiduciary responsibilities associated with its grant and subrecipient contracts to ensure these dollars serve these communities. HRSA must move beyond the language of ‘allowable’ uses to ‘expected integration of best practices.’
In many situations, we have been willing and able to confront harsh environments. Indeed, we recognize the need to be present in the spaces where political forces wish to silence us. However, Florida has crossed a line in becoming hostile to the very existence of Black and Brown and Immigrant and Transgender people, those same communities most affected by HIV. The people who enacted these hateful laws were motivated by hateful politics; our response is motivated by concern for the people we’re charged with representing in our community…many of whom feel silenced. This is a line which we cannot cross and still consider ourselves as living the values we espouse.
We came to the difficult decision that neither the ADAP Advocacy Association or Community Access National Network will host any advocacy or educational event in the state of Florida. We will continue to support local advocates and people living with HIV residing in the state, including scholarship support for intrastate travel by local advocates. We will continue to offer analysis on the state's activities. But we will not ask advocates from outside of the state to risk their mental health or physical safety to travel to the state.
Special Interests Favor S.4395, but Patients Oppose It...Here's Why
This blog post is a collaborative piece, co-written by Brandon M. Macsata, CEO of ADAP Advocacy Association, and Jen Laws, CEO of Community Access National Network.
The very first words of the Ryan White HIV/AIDS Treatment Extension Act of 2009 read, “An Act to amend title XXVI of the Public Health Service Act to revise and extend the program for providing life-saving care for those with HIV/AIDS.” These words reflect the true legislative intent of the Act, which is to provide life-saving care and treatment for people with HIV/AIDS (PLWHA). For over thirty years, these words have represented a contract between our government and PLWHA, reflecting a commitment to patients. The Ryan White HIV/AIDS Program (RWHAP), as the payor of last resort, has literally served as the only lifeline for hundreds of thousands of patients in some of the most marginalized communities. That is why the ADAP Advocacy Association and the Community Access National Network (CANN) have led a national advocacy campaign to thwart any effort to undermine the legislative intent.
A proposed bill, S.4395 (otherwise known as the "Ryan White PrEP Availability Act"), would, for the first time in the 32-year history of this life-saving contract, open the Act to divert programmatic funding from PLWHA to people who are not living with HIV. The legislation is not only ill-conceived, it is potentially very dangerous. The special interests behind this legislation, as well as their inside-the-beltway lobbying tactics, do not reflect the general sense of the much broader HIV patient advocacy community.
In fact, nearly 100 national, state, and local organizations joined the ADAP Advocacy Association and Community Access National Network in submitting a sign-on letter to Congress expressing the HIV patient advocacy community's collective concerns over the legislation. The sign-on letter was sent to Chair and Ranking Member of the Senate Committee on Health, Education, Labor & Pensions (HELP), Chair and Ranking Member of the House Committee on Energy & Commerce (E&C), and the Co-Chairs of the Congressional HIV/AIDS Caucus. Several of these offices applauded our efforts upon acknowledging receipt of the letter.
David Pable, who has been deeply embedded in South Carolina's patient advocacy community, expressed strong sentiments against the legislation. Pable said, "For almost 20 years, Ryan White has been a lifeline for me, and it was truly the safety net that saved my life. Ryan White-funded medical care, case management, and mental healthcare services have transform my life and the lives of countless others to survive and thrive." Pable's views are shared by nearly all PLWHA who learn about the potential danger lurking behind S.4395.
Over the years, Pable had the opportunity to be involved in many planning meetings for prevention services, including the need for an adequate PrEP program with dedicated funding. According to Pable, never in any of those meetings was it discussed as a good idea to funnel funding from the Ryan White Program to pay for PrEP. "Treatment, care and prevention make up three sides of the triangle," he said. "Together they each hold up the other, but take one piece away to support the other and eventually it will all fall apart."
S.4395 would authorize the Health Resources & Services Administration (HRSA) to divert already limited resources away from providing care and treatment for PLWHA. The legislation reads, in part, "Any eligible area, State, or public or private nonprofit entity that receives a grant under part A, B, C, or D may use program income received from such a grant to provide to individuals who are at risk of acquiring HIV... drugs and biological products for pre-exposure prophylaxis (PrEP)... medical, laboratory, and counseling services related to such drugs and biological products...and referrals and linkages to appropriate services for the prevention of HIV."
The legislation is extremely ill-advised for numerous reasons. Amending the Ryan White Program (Pub.L. 101-381) would:
Open-up the law, (which is currently unauthorized) and thus subject it to potentially harmful changes in a hyper-partisan political environment.
Change the purpose of the law, in that the purpose of the Ryan White Program is serving people living with HIV/AIDS.
Create yet another access barrier for the approximately 400,000 PLWHA who are not in care.
Further isolate PLWHA who are already disproportionally impacted by homelessness, hunger, substance use disorder, and undiagnosed and/or untreated mental health conditions.
Impede Ending the HIV Epidemic's efforts to both increase enrollment and expand services for low-income PLWHA, especially since discretionary funding is already limited.
Unfortunately, special interests continue to push false narratives in their efforts to shove the harmful legislation through the Congress. Probably one of the most egregious claims, “The bill’s intent and text doesn’t take money from people living with HIV.” This is false!
Indeed, legislative language reads, “To allow grantees under the HIV Health Care Services Program to allocate a portion of such funding for services to individuals at risk of acquiring HIV.” While subsection “B” of the legislation entitles the program as “voluntary” and to not allow federal grant dollars for the use of funding PrEP or PrEP services, it would allow federal grant dollars to be used for referrals – explicitly providing funding for people not living with HIV.
Photo Source: oncnursingnews.com
More concerning, special interests supporting the legislation conflate programmatic revenue as not grant dollars, as a somehow meaningful distinction. There is no difference in this distinction because each funded RWHAP recipient and subrecipient is required under current law to use their programmatic revenue to support providing services included in the grant – for people living with HIV. The design of these programs are significantly dependent on revenues generated from the 340B Drug Discount Program (340B) in order to meet the goals outlined in each of the grants.
And that gets to the heart of the issue here. 340B's intent was “to stretch scarce federal resources as far as possible, reaching more eligible patients and providing more comprehensive services.” The program, amid much criticism, allows federal grants funding public health programs count on 340B revenues in order to show they can operate a sustainable program.
Let's be clear: S.4395 would divert RWHAP programmatic revenues – including 340B dollars – away from providing services and supports to PLWHA who are living at or below 400% of the federal poverty level (the income threshold for qualifying as eligible for receiving RWHAP funded services). It is important to remember that more than 50% of the patients receiving care from the State AIDS Drug Assistance Program are living at or below 100% of the federal poverty level. More than 250,000 patients, or approximately one quarter of all the estimated people living with HIV in the United States are earning less than $13,000 per year.
Kathie Hiers, President & CEO of AIDS Alabama argued, "The HIV community needs to get its act together around funding for PrEP. We have been told by the Director of the Office of National AIDS Policy that our messaging is not cohesive. At AIDS Alabama, we understand that stable PrEP programs are absolutely necessary if we ever hope to end HIV as an epidemic. However, raiding the Ryan White Program to fund prevention is not the answer, particularly as the needs of an aging HIV-positive population continue to grow."
As it stands, gaps in care still remain for too many marginalized communities. It isn't uncommon for patients to fall out of care because they have to prioritize work, or child care, or buying food, or finding affordable housing, or finding transportation. Funding to meet the needs for these patients is already stretched way too thin and the current inflationary pressures have only made things harder for far too many PLWHA. There are tens of thousands of people living with HIV who have no roof over their heads when they try to find a safe spot to sleep tonight.
Photo Source: debralmorrison.com
Robbing Peter to pay Paul is not the solution to funding HIV prevention efforts in the United States. A better option to meet the needs of people who would benefit from PrEP, and that is additional HIV prevention funding. This approach would allow patient choice in medicines and support for ancillary services, provider education and outreach. Additionally, HIV prevention funding could be directed to communities that are most in need of prevention medicines and services, thereby providing more equitable access. This approach would also use and could strengthen the existing HIV prevention infrastructure.
One local health department official (who asked to remain anonymous) in Florida said the people behind the legislation did not understand the nuances between funding for HIV prevention and HIV treatment. We couldn't agree more!
The HIV+Hepatitis Policy Institute's Carl Schmid summarized, "It's not an issue of not wanting clinics that receive Ryan White Program funding to be engaged in PrEP, we think they are the perfect places for PrEP to be delivered. It is an issue of taking funding generated from caring and treating for people living with HIV away from the intended purpose of the Ryan White Program – to provide for people living with HIV. With so many people with HIV living longer, who are not in care or have fallen out of care, you would think that these Ryan White grantees would devote that money to people who are living with HIV, as it was intended."
With more than a decade of science to back the position that effectively treating PLWHA, ensuring viral suppression both empowers positive health outcomes for PLWHA and prevents new transmissions. One of the most startling and, frankly, concerning shifts in the public policy conversation regarding Ending the [domestic] HIV Epidemic is a move away from focusing on the needs of PLWHA in favor of PrEP. The policy issues at hand, including the necessary funding, should not be proposed as an “either/or” situation, but an “and” situation. The same things that make a person vulnerable to contracting HIV are the same things that are killing people already living with HIV.
While the U.S. Centers for Disease Control and Prevention (CDC) 2020 Surveillance data found 70% of white PLWHA were virally suppressed, only 60% of their Black/African American peers were virally suppressed. Additionally, while the U.S. Department of Housing and Urban Development (HUD) reported a general homelessness rate across the country as about 0.2% of the population, the CDC’s 2019 data found that PLWHA among communities of color were experiencing homelessness at a rate of 11%. It cannot be understated how the power RWHAP dollars hold to address these disparities specifically affecting patients. Failing to do so not only betrays the contract at the center of the legislative intent, it perpetuates injustices levied against our peers, our family, and our community. Raiding precious dollars from this program is nothing short of consenting to the unjust neglect of our communities.
Said Murray Penner, U.S. Executive Director for Prevention Access Campaign: "The Ryan White Program is crucial for people living with HIV, providing treatment and supportive services to keep people healthy and undetectable so they will not sexually transmit HIV. With over 400,000 people living with HIV in the U.S. who are not virally suppressed, there is significant unmet need for additional services. S.4395 would move money out of the Ryan White Program, potentially leaving people without the crucial treatment and services that keep them healthy and prevent new transmissions. Ensuring that the Ryan White Program is fully funded is critical for us to improve the quality of life for people living with HIV and thus improve our country's viral suppression rate and help us end the HIV epidemic."
A cornerstone of the HIV patient advocacy community's success over the last 40 years has been its desire to come together for a common purpose, which has centered around the notion of do no harm! S.4395 and the special interests and inside-the-beltway lobbyists pushing it have failed to meet that test. Raiding Ryan White programmatic funding for PrEP would negatively impact patients. Trying to authorize or amend an already underfunded program, when there is still so much unmet need in its originally intended population, undermines the goals of the program. If we try to be everything to everyone, we will end up failing on all fronts. The powers that be in Congress have assured us that this legislation "ain't going anywhere" this year!
European Commission Approves Once Every 6 Month Supplemental HIV-Treatment
Last week, the European Commission (EC) approved use of Gilead Sciences’ investigational medication, lenacapavir (branded as Sunlenca), for use in treatment for people whose HIV is highly treatment resistant. Lenacapvir has been closely watched by advocates because of great anticipation regarding injectable and otherwise long-acting agents (LAAs) and because of its exceptionally long half-life in the body, providing efficacy for about 6 months from just one subcutaneous injection, providing a potential for the longest acting agent to hit the market thus far.
Lenacapavir also stands out as a first-in-class product because it works in a way that no other antiretroviral medication works. Offering a novel mechanism of action, binding the shell (capsid) surrounding viral genetic material that highjacks our own cells in HIV reproduction, capsid inhibitors will work against HIV in multiple areas of the virus’ life cycle. And because this is a tool we’ve never used before, it’s ideal for meeting the treatment needs of people living with highly resistance HIV – the virus doesn’t recognize the medication and has not yet found a way around it. Because HIV is highly adaptable, that lack of resistance may be short-lived once broad reach of the product is made available but excitement remains for a novel product, especially for people who have developed such resistance that options of effective care have diminished to near zero.
Gilead Sciences is awaiting a decision from the United States’ Food and Drug Administration (FDA) on its recently resubmitted “New Drug Application” (NDA), after the agency declined to accept the NDA due to issues with the glass vials used for the injectable form of the medication. The vial issue has since been fixed and research resumed for use of lenacapavir as a supplement to “background” treatment, first-line treatment, and as pre-exposure prophylaxis (PrEP). At the end of July, the FDA provided Gilead with a December 27, 2022 prescription drug user fee date. This is one of the final steps in an initial new drug approval. (Briefly, the Prescription Drug User Fee Act funds a massive portion of the FDA and those medications which pose potential benefit to public health may receive an expedited fee date; HIV advocates historically championed the act as a massive “win” in the early 90’s when introducing this funding scheme sped up their processes to make oncology and HIV medications more readily accessible.)
Patients who may hear about “once every 6-month treatment) should be kindly reminded lenacapavir will serve only as a supplement to other therapies – administering the medication is required to be done by a provider and patients will still need to remain adherent to their existing treatment regimen. HIV medications for treatment need between 2 and 3 different combinations of medications and PrEP only needs 1. As such, there may be a disparity in lenacapavir as PrEP and Sunlenca as treatment in terms of uptake because, while lenacapavir won’t require maintenance of other medications, Sunlenca as treatment will require patients to continue to take the oral medications that are “optimized” with Sunlenca acting almost like a “soft reset” for patients.
Operationalizing will remain an issue, especially for state ADAP Program’s that have not fully implemented access to cabotegravir, in terms of injectable medication management and for arguments that frame investing in successful patient care as something that requires a “cost consideration”. Patient advocates should be well-prepared to defend a desire to see this product come to their patients as those patients so desire it.
2022: New Beginnings, New Changes
The Community Access National Network (CANN) ushers in a new beginning with the 2022 New Year, evidenced not only by the changing of the guard with our new President & CEO, but also with some important programmatic changes with our organization. We felt it important to share these changes with you.
Our weekly blog, previously branded as the HEAL Blog (Hepatitis Education, Advocacy & Leadership), is being repurposed to serve our broader mission “to define, promote, and improve access to healthcare services and supports for people living with HIV/AIDS and/or viral hepatitis through advocacy, education, and networking.” As such it is now the CANN Blog, and its areas of interest will focus on HIV/AIDS, viral hepatitis, substance use disorder, harm reduction, patient assistance programs (PAPs), Medicare, Medicaid, and the ongoing Covid-19 pandemic and its impact on public health. In keeping with the desire to monitor broader public health-related issues and appropriately engage stakeholders, our CANN Blog will be disseminated to a larger audience. Therefore, some of you may notice one more email in your inbox each Monday morning since we’re employing our general listserv to share the blog posts. It is our hope that you’ll deem the added email of value and thus maintain yourself on our listserv.
Additionally, our acclaimed HIV/HCV Co-Infection Watch will also be shared with our general listserv. But don’t worry, it only means one additional email each quarter! The HIV/HCV Co-Infection Watch offers a patient-centric informational portal serving three primary groups - patients, healthcare providers, and AIDS Service Organizations. The quarterly Watches are published in January, April, July, and October.
In 2022, our Groups will also be more active. Since 1996, our National ADAP Working Group (NAWG) has served as the cornerstone of CANN’s advocacy work on public policy. Whereas NAWG will continue to engage our HIV/AIDS stakeholders with monthly news updates, we will also convene periodic stakeholder meetings to discuss important issues facing the HIV community. Likewise, our Hepatitis Education, Advocacy & Leadership (HEAL) Group has served as an interactive national platform for the last decade on relevant issues facing people living with viral hepatitis. Periodic stakeholder meetings to discuss important issues facing the Hepatitis community will now complement the HEAL monthly newsletter. If you would like to join either the NAWG or HEAL listserv, then please do so using this link.
CANN will also launch its 340B Action Center this year. It is designed to provide patients with content-drive educational resources about the 340B Drug Discount Program and why the program matters to you. The importance of the 340B Program cannot be under-stated, and CANN remains committed to taking a balanced “money follows the patient” approach on the issues facing the program and advocating for needed reforms.
Finally, like most advocacy organizations, CANN is constantly evaluating whether it is safe (or not) to host in-person stakeholder meetings. Covid-19 has changed the advocacy landscape. Over the last two years our two signature meetings (Community Roundtable and Annual National Monitoring Report on HIV/HCV Co-Infection) have been hosted virtually, rather than in-person. CANN is taking a “wait and see” approach on how best to proceed in 2022 with these events. We will keep you apprised of our decision.
As we close the door on 2021 and open it for 2022, CANN looks forward to working with all of its community partners, industry partners, and you!
2021: A Year in Reflection
The end of 2021 is upon us and that makes this a timely opportunity to reflect on the work by the Community Access National Network (CANN). During an exceedingly busy news cycle, we have published fifty blogs (including this one) on a variety of topics ranging from the latest on policy and regulatory issues, as well as some personal perspectives. Our HIV-HCV Coinfection Watch and our Annual Monitoring Report tracked Hepatitis C (HCV) therapies covered under the State AIDS Drug Assistance Programs, Medicaid, Veterans Administration, as well as patient access via patient assistance programs, and other relevant news items affecting our patient community. We also conducted a community roundtable seeking to highlight the impacts of Covid-19 on public health programs aimed at addressing HIV, HCV, and substance use disorder (SUD).
Notably, CANN published the following six-part series designed to educate patients on various aspects of the 340B Drug Discount Program:
· A Patient’s Guide to 340B: Why the Program Matters to You
· A Patient’s Guide to 340B: Why Transparency Matters to You
· A Patient’s Guide to 340B: Why Accountability Matters to You
· A Patient’s Guide to 340B: Why the Decline in Charity Care Matters to You
· A Patient’s Guide to 340B: Why the Middlemen Matters to You
· A Patient’s Guide to 340B: Why Program Reform Matters to You
With Congress engaged in high-conflict communication, to abuse a euphemism, navigating public policy developments and pertinent issues to patients can be challenging. CANN remains committed to being an essential source of two-way communication, information, and education wherein patients write the narrative driving policy reforms and priorities. In this, we are ever grateful to the patients and caretakers who have engaged with us at every turn. Your stories matter and you are not alone in your experiences.
The diverse partnerships behind this work are critical to our success and as we end the year, we want to offer our gratitude to these essential partnerships, ranging from other patient advocacy organizations, public health associations, and industry partners.
The issues affecting our public health space of patient advocacy have not relented this year. Covid-19 has only emphasized the need to ensure these programs are effective and efficient while also highlighting the existing weaknesses and strengths of these programs. To be clear, the structural and pervasive drivers of health disparities have been named; racism, sexism, classism, ableism, and all other biases which reflect a moral justification for out ethical failings must be addressed in tandem with policy changes and adequate public health program funding in order for us to succeed in these fights for patient lives. Health equity cannot be meaningfully segregated from the policy mechanisms in which these disparities have survived in the face of another pandemic – when our collective awareness of these inequities and leverage to progress on these issues should have been their strongest and yet were not.
It’s with these things in mind, we want to leave you with the enduring sentiment that next year offers us yet another opportunity to approaches these challenges with fresh eyes and fresh ideas. We are indeed stronger together and we sincerely look forward to working with you all to move closer in realizing a world of greater access to care, fewer and smaller health disparities, and, ultimately, a more fair and loving environment in which to live our lives and raise our families.
Author’s note: I often end certain professional meetings with telling my colleagues “Love ya’ll”. It’s a sentiment I mean to depths of my soul. I am fortunate to work with some of the most amazing people in the world – folks who share an unbridled commitment to improving the lives of those around them. It’s from this same space I wish to offer each of you reading this a moment to breathe and the same open heartedness. I want to leave you all with a short story that has shaped me in more ways than I can count, The Perfect Heart, and an encouragement to tell someone you love them as soon as you can. May this next year be gentler with us all and find us giving away more pieces of our hearts.
Jen’s Half Cents: Stepping into the Shadow of a Giant
I’ve often tried to distinguish between the sense of grief and shock that follows the death of a hero. Emotions are complex…layered especially when it comes to grief and the amount of work that’s involved in mourning and appropriate appreciation for a person’s life. When a young person dies unexpectedly, the sense of shock layered onto grief is exorbitant. That shock is expected to be less so when a person of advanced age moves on from this plane. This sentiment is not to disregard the tragedy of loss but to distinguish between “heavy” and “sharp” pains.
When William “Bill” Arnold passed on September 29th, I didn’t expect to feel the deep sense of shock that struck my heart. Tragedy, yes, because losing the presence of this titan is indeed a tragic loss for the community of advocates his work and passion shaped. But the shock was unexpected. There’s this thing that happens when admiration is a core part of one’s perception of a person – regardless of age, the relationship seems to defy necessary acknowledgments of nature even if, as was the case with Bill, the subject of said admiration challenged the admirer to not forget. In writing my second “Half Cents” this year, Bill appreciated the emphasis on needing to shore up the advocacy pipeline. After all, the blog was based in part on the very first discussion he and I shared. He cautioned, however, “there’s an obvious target here”, referring to himself. A stark reminder of his age. I think…I should have taken more note of the comment and in retrospect I regret not seeking more of an opportunity to discuss how he felt about the piece and what it meant for him, personally.
Writing this blog is a particular challenge in that few words can express the deeply complex set of feelings I’ve had to navigate since Bill’s death. The last in-person conversation Bill and I had was outside of a restaurant, after a fireside chat hosted by CANN’s sister organization, ADAP Advocacy Association in early December of 2019. As Bill and I were among the few smokers in the crowd, I enjoyed spending time with him outside, even in the bitter cold New Jersey had to offer that evening. We didn’t talk about recent politics or the upcoming election or even particular policy issues the event focused on earlier in the day, as was more common of our “smoker’s chats”. Instead, we talked about his childhood and the quite remarkable, yet humble life Bill lead. I like to remind advocates we should “bleed a little” in our work because the lives we seek to impact and the stories that drive this work are the emotional blood bonds of effective advocacy. It is unjust to expect patients to share the intimate aspects of their lives and health and to not return the favor. And in those moments, what Bill chose to share with me felt less like bleeding a bit of himself and more like welcoming me into his home. I don’t know how to reconcile that quite yet.
The greatest tragedy of death, however, is the world does not stop for grief. Things still need to be done in order for the world to function, work does not stop piling up, decisions must be made in order to not compound the difficulty of an already difficult time. In that respect, CANN’s general consultant, Brandon M. Macsata was tasked with making a recommendation of succession of Bill’s duties to CANN as President and Chief Executive Officer to the organization. This was not an easy thing. Bill had been a mentor and friend to Brandon for more than 20 years and the enormity of the moment weighed on him. He wanted to fulfill his responsibility to the organization and do justice to the Bill’s legacy, as did CANN’s Board of Directors. In this space, while we may not all always be friends, we are all comrades in a fight for improving access to care and thus the lives of those around us, in particular, the most vulnerable of our shared communities. This decision was both professional and personal, as it should be, in such intimate work.
So, when the Board of Directors, through Brandon, approached me to ask if I would be interested in assuming the role of President and Chief Executive Officer, stepping into Bill’s shadow, I had to take a moment. “These are mighty big shoes to fill”, I’d say before expressing some trepidation, not at my skill or ability, but because of an earnest desire to ensure Bill’s legacy would be appropriately honored - the Board, our patient community, and our partners would be proud of the work that follows. In expressing confidence and navigating the decision-making process, CANN’s board members placed an emphasis on both skill and temperament, a need to focus on policy changes from the patient perspective, and for the next chapter of leadership for the organization to balance these qualities and ideals.
“You and Bill are cast from the same mold”, will forever be one of the greatest compliments I will ever receive. It’s one I hold dear to my heart and the sentiment provides me a laser focus the mission at hand.
The outpouring of support CANN has received, both in offering condolences and in appointing me to the role of President and Chief Executive officer, has been incredible. While changes are certain for CANN’s future, the organization will continue with the same goals it was founded upon and has served for the 25 years; “defining, promoting, and improving access to healthcare services and supports for people living with HIV/AIDS and/or viral hepatitis through advocacy, education, and networking.”
A Patient’s Guide to 340B: Why Program Reform Matters to You
***This is the final report in a six-part series to educate patients about the 340B Drug Pricing Program***
The 340B Drug Pricing Program has no doubt added benefit for patients and providers, alike. The measure of this benefit, however, is shrouded by uncertainty over the lack of transparency and accountability, decline in hospital charity care, as well as the explosive middleman growth in contract pharmacies and pharmacy benefit managers. Twenty-nine years after the program’s inception, it is now unclear to both regulators and patients, both qualitatively and quantitatively, if the Congressional intent is being met.
With all the noise around whether rebate programs might encourage pharmaceutical manufacturers to raise the cost of their products, there is no conversation on how those rebate dollars are used. The lack of the requisite transparency reporting among non-federal grantee covered entities participating in the 340B program makes it impossible to distinguish between anecdotal claims of abuses versus legitimate use of these rebate dollars to the benefit of patients.
These combined situations place the future of the 340B program at exceptional risk, if only by politicization of the national conversation on medication affordability alone. That national conversation churns now, as Congress debates drug pricing legislation. Aside from notorious stump speeches about the prices other countries pay for their medications, nowhere in these discussions do we talk about payers (insurers) and the middleman dictating the at-the-counter prices of medications realized by patients. The ongoing political debate is absent of the larger impacts on safety-net programs benefitting from 340B revenue and the impact on the poorest patients among us. Without clear guidance, all patients can come to expect is more squabbling among covered entities, drug manufacturers, hospitals, and regulators. It is this type of environment in which an idealistic program finds itself at risk.
Lawmakers have reasonably argued federal regulators have not demonstrated a particular need for additional regulatory powers because the Health Services and Resources Administration (HRSA) has not adequately flexed their current oversight muscle (…much less that such would be exercised efficiently). Therefore, regulatory interpretation should be updated, specifically regarding the patient definition, and possibly with further defining “low-income” for more clarity on who the program should benefit most. To the extent of “cracking open the legislation”, there is a singular area in which lawmakers from both sides and the Biden Administration agree: the issue of transparency in reporting. Earlier this year, the Biden Administration’s discretionary budget included an ask of Congress to specifically fund greater oversight and administration of 340B, explicitly including requirements on reporting of how non-grantee entities use these dollars. In this space, where few agreements can be made found, this is one area where legislators can and should move swiftly. The data generated by transparent reporting on use of these dollars is invaluable in evaluating the efficacy of 340B in benefitting patients or otherwise meeting the intent of the program.
To the extent HRSA may need more room for rulemaking, legislators desperately need extend rulemaking authority to include allowable uses for 340B dollars and clarity on the intent of the program. Federal grantees already have to report use of these dollars while other covered entities aren’t. With executives reaping in millions of dollars, reasonable people can grow concerned these dollars are being used to prop up the profiteering and personal enrichment administrators may be enjoying at the expense of employees providing care and patients themselves. Employees of federal grantees don’t generally get to enjoy much in the way of raises and their pay is not on par with the private sector. Hardware and software systems lag in terms of keeping up with modern technology. Sustaining non-revenue generating or underfunded patient benefit programs is absolutely something many entities enjoy as a use of their 340B dollars. There is no doubt these dollars can be used to patient benefit beyond directly sharing the savings with patients, though sharing the savings is the most direct means patients benefit from 340B. Putting guardrails on allowable uses of these dollars would serve well everyone touched by the program. Frankly, anyone fighting this transparency as a suggested method of shoring up 340B in meeting its intended purpose has something to hide and deserves closer scrutiny.
As an additional area of critical need to consider, for non-grantee covered entity hospitals, records of charity care and minimum realized values in served communities should be determinative for qualification to participate as a covered entity. The current calculation of disproportionate share hospitals as 340B participants or non-340B participants by the Government Accountability Office has shown a steadier and steeper decline of charity care among 340B hospitals than among non-340B hospitals. Additionally, hospitals carry the highest issuance of medical debt in the United States, disproportionately affecting low-income patients. Part of ensuring low-income patients get the most benefit from the discount drug program was and remains the ability to extend no- and low-cost care, writing off costs of providing that care, without punishing patients for having a need. If hospitals are to receive the benefit of this program, that same benefit should be extended to patients.
Lastly, in addressing the sheer size of the 340B discount drug program, the most significant areas of growth with questionable benefit to patients are among contract pharmacies. HRSA’s recognized this potential in commentary with its 2010 final rule only to realize those cautionary concerns and integrate guidance curbing the use and growth of contract pharmacies in the no-shelved 2015 “mega-guidance”. While the mega-guidance has been shelved, the abuse of the program by contract pharmacies has not abated. Among reducing the number of contract pharmacies a covered entity may make agreements with, and other geographic requirements, lawmakers and regulators should consider establishing market appropriate flat fees associated with services and a database of fees charged by pharmacy benefits managers, contract pharmacies, and third-party administrators, similar to the 340B ceiling price database established under the Office of Pharmacy Affairs Information System. A similarly situated claims hub would also allow for greater clarity in audits, assessment of potential duplicate discounts, and (if appropriately structured and compliant with patient privacy laws) detect potential diversion.
340B is a massive program which, arguably, has not yet been realized by much of the patient population. Not doing anything in this case doesn’t mean “keeping things status quo”, rather it means leaving the program open to attack, inefficiency, ineffectiveness, and abuse. We can and should do more to ensure patients are aware of the program, how the program is used by covered entities nearest to them, and how this critical support to federally funded health care programs might be impacted by additional health care policy reform efforts. If ensuring the health and well-being of the country is the priority of all players in this system, then its time patients know it.
For more information on the issues facing the 340B Program, you can access the Community Access National Network’s 340B Commission final report and reform recommendations here.
Sources:
Community Access National Network (February 2019). 340B DRUG DISCOUNT PROGRAM: The Issues Spurring Discussion, Stakeholder Stances and Possible Resolutions. 340B Commission Final Report. Retrieved online at https://docs.google.com/gview?url=http://www.tiicann.org/pdf-docs/2019_CANN_340B_Commission_Final-Report-v5_03-07-19.pdf&embedded=true