Watch 02: April 2023
The HIV/HCV Co-Infection Watch is a project of the Community Access National Network (CANN) designed to research, monitor and report on HIV and Hepatitis C (HCV) co-infection in the United States. The April 2023 Watch includes timely updates herein. To read the project disclaimer and/or methodology, CLICK HERE.
1. FINDINGS
The following is a summary of the key findings for April 2023:
AIDS Drug Assistance Programs:
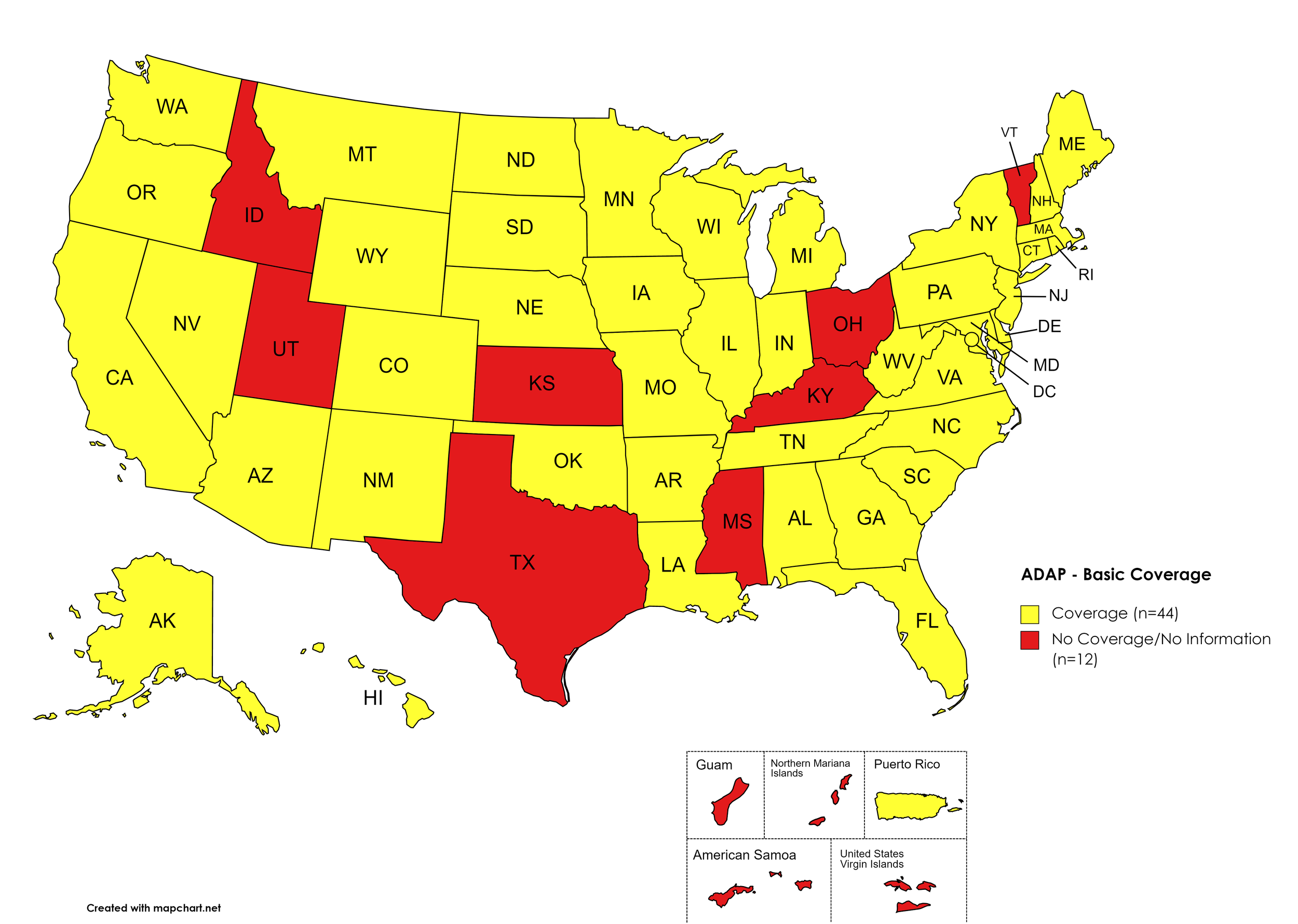
There are 56 State and Territorial AIDS Drug Assistance Programs (ADAPs) in the United States, 47 of which offer some form of coverage for Hepatitis C (HCV) treatment. Of those programs, 45 have expanded their HCV coverage to include the Direct-Acting Antiviral (DAA) regimens that serve as the current Standard of Care (SOC) for Hepatitis C treatment. Two (2) programs offer only Basic Coverage and 9 programs offer No Coverage. One (1) program covers only a single Direct-Acting Antiviral. Three (3) territories – American Samoa, Marshall Islands, and Northern Mariana Islands – are not accounted for in this data. A state-by-state Drug Formulary breakdown of coverage is included in the April 2023 Updates, with accompanying drug-specific maps in Figures 1 – 10.
Medicaid Programs:
There are 59 State and Territorial Medicaid programs in the United States, and data is represented for all fifty (50) states and the District of Columbia. As of October 01, 2016, all 50 states and the District of Columbia offer Expanded Coverage. A state-by-state PDL breakdown of coverage is included in the April 2023 Updates, with accompanying drug-specific maps in Figures 11 – 20.
Harm Reduction Programs:
Every State and Territory in the United States currently provides funding for low-income people living with substance abuse issues to enter state-funded rehabilitation services (National Center for Biotechnology Information, n.d.). Forty-four (44) States, the District of Columbia and three (3) Territories currently have Syringe Services Programs (SSPs) in place, regardless of the legality. Fifty (50) States and the District of Columbia have expanded access to Naloxone to avert opioid drug overdoses. Fifty (50) States and the District of Columbia have Good Samaritan laws or statutes that provide some level of protection for those rendering emergency services during drug overdoses. Forty-seven (47) States, the District of Columbia, and Guam make reporting to Prescription Drug Monitoring Programs (PDMPs) mandatory, requiring physicians and/or pharmacists to report prescriptions written or filled to a state agency for monitoring. Fifty (50) States and the District of Columbia have Opioid-Specific Doctor Shopping Laws preventing patients from attempting to receive multiple prescriptions from numerous physicians, and/or from withholding information in order to receive prescriptions. Forty-five (45) states and the District of Columbia mandate a Physical Exam Requirement in order for patients to receive a prescription for opioid drugs. Thirty-Five (35) states have in place an ID Requirement mandating that people filling opioid prescriptions present a state-issued ID prior to receiving their prescription. Forty-five (45) states and the District of Columbia require prescribing physicians to attend mandatory and continuing opioid prescribing education sessions. Forty-seven (47) states and the District of Columbia have Medicaid doctor/pharmacy Lock-In programs that require patients to receive prescriptions from a single physician and/or fill prescriptions from a single pharmacy. A state-by-state program breakdown is included in the April 2023 Updates, with accompanying drug-specific maps in Figures 21-29.
2. AIDS DRUG ASSISTANCE PROGRAMS (ADAPs) & HCV THERAPIES
Of the 56 respective State and Territorial ADAPs, only 8 (KS, KY, OH, UT, VT, GU, PW, VI) do not offer any coverage for HCV drug therapies. States whose formularies are not available on the state-run website have been checked against the most recent National Alliance of State and Territorial AIDS Directors (NASTAD) formulary database (last updated January 1, 2022). The data presented are current as of April 7, 2023.
April 2023 Updates:
Basic Coverage
States with Basic HCV Medications Coverage: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, IL, IN, IA, LA, ME, MD, MA, MI, MN, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OK, OR, PA, RI, SC, SD, TN, VA, WA, WV, WI, WY, D.C.
States without Basic HCV Medications Coverage: ID, KS, KY, MS, OH, TX, UT, VT
Territories with Basic HCV Medications Coverage: P.R.
Figure 1. April 2023 ADAP Coverage - Basic HCV Medications
Map Key: Yellow = Basic HCV Medication Coverage; Red = No Basic HCV Medication Coverage/No Information regarding Basic HCV Medication Coverage
Sovaldi
States with Sovaldi Coverage: AZ, CA, CO, GA, HI, IL, IN, IA, LA, ME, MD, MA, MN, NE, NV, NH, NJ, NM, ND, OK, OR, PA, SD, VA, WA, WI, WY, D.C.
States without Sovaldi Coverage: AL, AK, AR, CT, DE, FL, ID, KS, KY, MI, MS, MO, MT, NY, NC, OH, RI, SC, TN, TX, UT, VT, WV
Territories with Sovaldi Coverage: P.R.
Figure 2. April 2023 ADAP Coverage - Sovaldi
Map Key: Yellow = Sovaldi Coverage; Red = No Sovaldi Coverage/No Information regarding Sovaldi Coverage
Harvoni
States with Harvoni Coverage: AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, NE, NV, NH, NJ, NM, NC, ND, OK, OR, PA, SD, TN, VA, WA, WI, WY, D.C.
States without Harvoni Coverage: AL, AK, KS, KY, MO, MT, NY, OH, RI, SC, TX, UT, VT, WV
Territories with Harvoni Coverage: P.R.
Figure 3. April 2023 ADAP Coverage - Harvoni
Map Key: Yellow = Harvoni Coverage; Red = No Harvoni Coverage/No Information regarding Harvoni Coverage
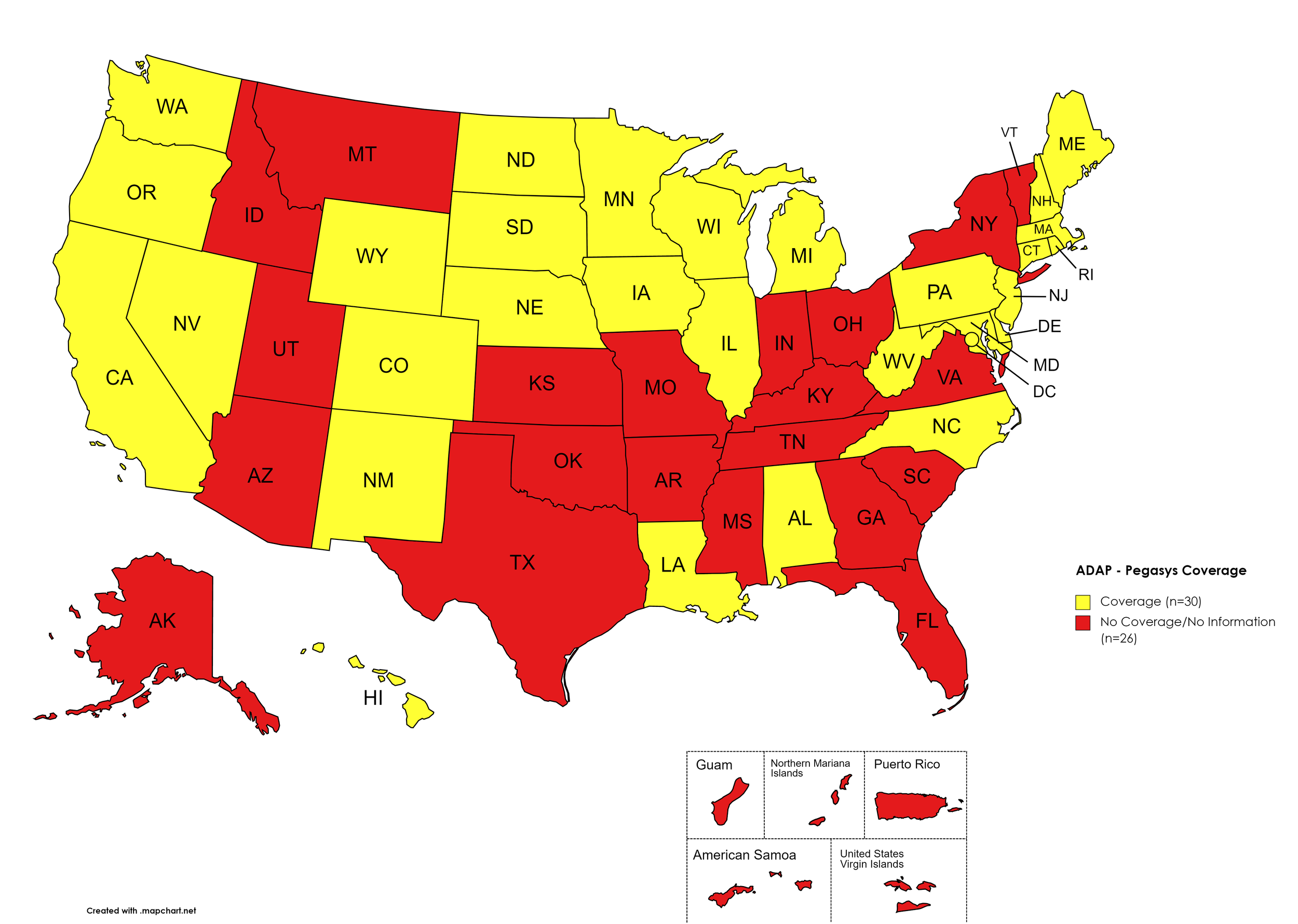
Zepatier
States with Zepatier Coverage: AL, AZ, AR, CA, CO, FL, GA, HI, IL, IA, LA, ME, MD, MA, MI, MN, MS, NE, NV, NH, NJ, NM, NY, NC, ND, OR, PA, SD, VA, WA, WV, WI, WY, D.C.
States without Zepatier Coverage: AK, CT, DE, ID, IN, KS, KY, MO, MT, OH, OK, RI, SC, TN, TX, UT, VT
Territories with Zepatier Coverage: P.R.
Figure 4. April 2023 ADAP Coverage - Zepatier
Map Key: Yellow = Zepatier Coverage; Red = No Zepatier Coverage/No Information regarding Zepatier Coverage
Epclusa
States with Epclusa Coverage: AZ, AR, CA, CO, CT, FL, GA, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, MO, NE, NY, NV, NH, NJ, NM, ND, OR, PA, SD, TN, VA, WA, WI, WY
States without Epclusa Coverage: AL, AK, DE, KS, KY, MT, NC, OH, OK, RI, SC, TX, UT, VT, WV, D.C.
Territories with Epclusa Coverage: P.R.
Figure 5. April 2023 ADAP Coverage - Epclusa
Map Key: Yellow = Epclusa Coverage; Red = No Epclusa Coverage/No Information regarding Epclusa Coverage
Vosevi
States with Vosevi Coverage: CA, CT, FL, HI, ID, IL, IN, IA, LA, MD, MA, MN, NE, NV, NH, NJ, NM, ND, OR, SD, TN, WA, WY
States without Vosevi Coverage: AL, AK, AZ, AR, CO, DE, GA, KS, KY, ME, MI, MS, MO, MT, NY, NC, OH, OK, PA, RI, SC, TX, UT, VT, VA, WV, WI, D.C.
Territories with Vosevi Coverage: P.R.
Figure 6. April 2023 ADAP Coverage - Vosevi
Map Key: Yellow = Vosevi Coverage; Red = No Vosevi Coverage/No Information regarding Vosevi Coverage
Mavyret
States with Mavyret Coverage: AL, AZ, AR, CA, CO, CT, FL, GA, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OR, PA, SD, TN, VA, WA, WV, WI, WY, D.C.
States without Mavyret Coverage: AK, DE, KS, KY, OH, OK, RI, SC, TX, UT, VT
Territories with Mavyret Coverage: P.R.
Figure 7. April 2023 ADAP Coverage - Mavyret
Map Key: Yellow = Mavyret Coverage; Red = No Mavyret Coverage/No Information regarding Mavyret Coverage
Pegasys
States with Pegasys Coverage: AL, CA, CO, CT, DE, HI, IL, IA, LA, ME, MD, MA, MI, MN, NE, NV, NH, NJ, NM, NC, ND, OR, PA, RI, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Pegasys Coverage: AK, AZ, AR, FL, GA, ID, IN, KS, KY, MS, MO, MT, NY, OH, OK, SC, TN, TX, UT, VT, VA
Territories with Pegasys Coverage: None/Unknown
Figure 8. April 2023 ADAP Coverage - Pegasys
Map Key: Yellow = Pegasys Coverage; Red = No Pegasys Coverage/No Information regarding Pegasys Coverage
Harvoni (generic)
States with Harvoni (generic) Coverage: AZ, AR, CA, CO, CT, FL, IL, IA, ME, MD, MA, MN, MS, NE, NV, NH, NJ, NM, NC, ND, OK, OR, PA, SD, TN, WA, WI, WY, D.C.
States without Harvoni (generic)Coverage: AL, AK, DE, GA, HI, ID, IN, KS, KY, LA, MI, MO, MT, NY, OH, RI, SC, TX, UT, VT, VA, WV
Territories with Harvoni (generic) Coverage: P.R.
Figure 9. April 2023 ADAP Coverage - Harvoni (Generic)
Map Key: Yellow = Harvoni (Generic) Coverage; Red = No Harvoni (Generic) Coverage/No Information regarding Harvoni (Generic) Coverage
Epclusa (generic)
States with Epclusa (generic) Coverage: AZ, AR, CA, CO, CT, FL, IL, IN, IA, ME, MD, MA, MN, MS, MO, NE, NV, NH, NJ, NM, ND, OR, PA, SD, TN, WA, WI, WY, D.C.
States without Epclusa (generic) Coverage: AL, AK, DE, GA, HI, ID, KS, KY, LA, MI, MT, NY, NC, OH, OK, RI, SC, TX, UT, VT, VA, WV
Territories with Epclusa (generic) Coverage: P.R.
Figure 10. April 2023 ADAP Coverage - Epclusa (generic)
Map Key: Yellow = Epclusa (generic) Coverage; Red = No Epclusa (generic) Coverage/No Information regarding Epclusa (generic) Coverage
April 2023 Notes:
States with Open Formularies: IL, IA, MA, MN, NE, NH, NJ, NM, ND, OH, OR, WA, WY
N.B. – Although Ohio is listed by NASTAD as having an open formulary, both NASTAD’s ADAP Formulary Database and Ohio’s ADAP website indicates that the state does not offer any treatment for HCV.
N.B. – Although North Dakota has adopted an open formulary, they provide only co-pay and deductible assistance for HCV medications.
N.B. – Wyoming's ADAP Open Formulary document, the following disclaimer related to HCV is made: Hepatitis C treatment medications (i.e. Harvoni, Sovaldi, Ribavirin, Zepatier, Epclusa) must be prior authorized. To be eligible, clients must have applied for prior authorization from their insurance plan and the WY ADAP Hepatitis C Treatment checklist must be completed and signed by the provider and client.
Colorado offers five coverage options – Standard ADAP, HIV Medical Assistance Program (HMAP), Bridging the Gap Colorado (BTGC), HIV Insurance Assistance Program (HIAP), and Supplemental Wrap Around Program (SWAP). ‘Yes’ indications in Figure 1. for Colorado denote that at least one of these programs offers coverage for each respective drug. The Standard ADAP Formulary covers medications only if funds are available to do so.
Louisiana’s ADAP (Louisiana Health Access Program – LA HAP) offers two coverage options – Uninsured (Louisiana Drug Assistance Program – L-DAP) and Insured (Health Insurance Program – HIP). HIP pays for the cost of treatment only if the client’s primary insurance covers the drug under its formulary.
Georgia’s ADAP notes the following: “Georgia ADAP Hepatitis C Program is currently on HOLD until future funding is available. Please utilize Patient Assistance Programs (PAP’s) for Hepatitis C medications.”
Texas ADAP has eliminated all HCV coverage.
3. MEDICAID PROGRAMS & HCV THERAPIES
All 50 states and the District of Columbia continue to offer some form of HCV coverage. All 50 states and the District of Columbia have expanded their Preferred Drug Lists to include at least one HCV Direct Acting Agent (DAA).
April 2023 Updates:
Basic Coverage
States with Basic HCV Medications Coverage: AZ, AK, AR, CA, CO, CT, DE, FL, GA, HI, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OR, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Basic HCV Medications Coverage: AL, ID, KS, MO, OK, SC, VA, WY
Figure 11. April 2023 Medicaid Coverage - Basic HCV Medications
Map Key: Blue = Basic HCV Medication Coverage; Yellow = No Basic HCV Medication Coverage/No Information regarding Basic HCV Medication Coverage
Sovaldi
States with Sovaldi Coverage: AR, CA, CO, DE, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WI, D.C.
States without Sovaldi Coverage: AL, AK, AZ, CT, FL, IA, NH, NM, OK, OR, SC, VA, WV, WY.
Figure 12. April 2023 Medicaid Coverage - Sovaldi
Map Key: Blue = Sovaldi Coverage; Yellow = No Sovaldi Coverage/No Information regarding Sovaldi Coverage
Harvoni
States with Harvoni Coverage: AL, AR, CA, CO, DE, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Harvoni Coverage: AK, AZ, CT, FL, IA, NM, OK, OR, SC, VA, WY.
Figure 13. April 2023 Medicaid Coverage - Harvoni
Map Key: Blue = Harvoni Coverage; Yellow = No Harvoni Coverage/No Information regarding Harvoni Coverage
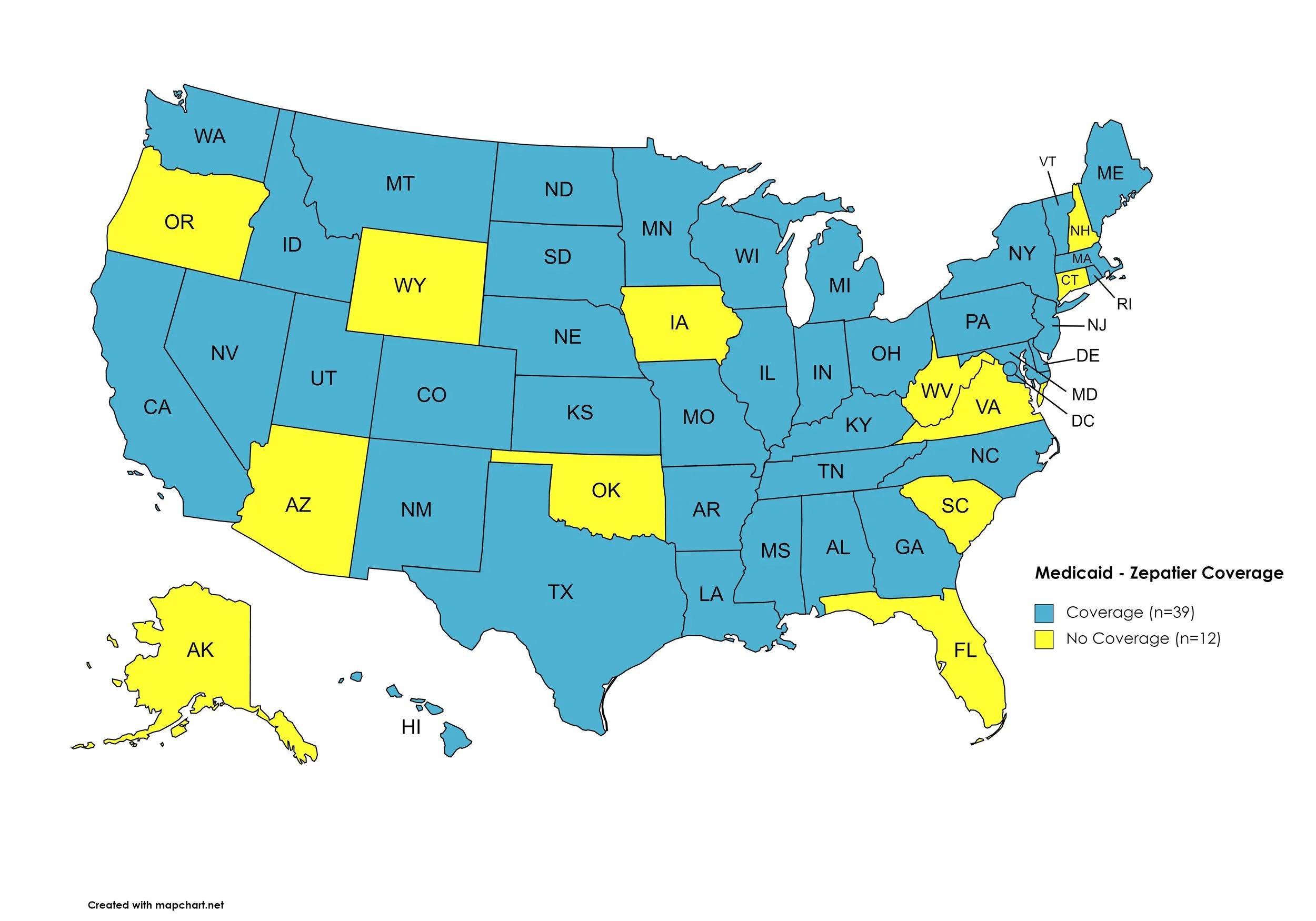
Zepatier
States with Zepatier Coverage: AL, AR, CA, CO, DE, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WI, D.C.
States without Zepatier Coverage: AK, AZ, CT, FL, IA, NH, NM, OK, OR, SC, VA, WV, WY.
Figure 14. April 2023 Medicaid Coverage - Zepatier
Map Key: Blue = Zepatier Coverage; Yellow = No Zepatier Coverage/No Information regarding Zepatier Coverage
Epclusa
States with Epclusa Coverage: AL, AR, CA, CO, DE, GA, HI, IL, IN, KS, KY, LA, MA, ME, MI, MN, MS, MO, MT, NV, NH, NJ, NM, NY, NC, ND, OH, OR, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Epclusa Coverage: AK, AZ, CT, FL, ID, IA, MD, NE, OK, SC, VA, WY.
Figure 15. April 2023 Medicaid Coverage - Epclusa
Map Key: Blue = Epclusa Coverage; Yellow = No Epclusa Coverage/No Information regarding Epclusa Coverage
Vosevi
States with Vosevi Coverage: AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NY, NC, ND, OH, PA, RI, SC, SD, TN, TX, UT, VT, WA, WI, D.C.
States without Vosevi Coverage: AL, AK, AZ, NM, OK, OR, VA, WV, WY.
Figure 16. April 2023 Medicaid Coverage - Vosevi
Map Key: Blue = Vosevi Coverage; Yellow = No Vosevi Coverage/No Information regarding Vosevi Coverage
Mavyret
States with Mavyret Coverage: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
Figure 17. April 2023 Medicaid Coverage - Mavyret
Map Key: Blue = Mavyret Coverage; Yellow = No Mavyret Coverage/No Information regarding Mavyret Coverage
Pegasys
States with Pegasys Coverage: AK, AZ, CA, CT DE, FL, GA, HI, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MS, MT, NE, NV, NH, NJ, NM, NY, NC, OH, OR, PA, RI, SD, TN, TX, VT, WA, WV, WI, D.C.
States without Pegasys Coverage: AL, AR, CO, ID, KS, MO, ND, OK, SC, UT, VA, WY
Figure 18. April 2023 Medicaid Coverage - Pegasys
Map Key: Blue = Pegasys Coverage; Yellow = No Pegasys Coverage/No Information regarding Pegasys Coverage
Harvoni (generic)
States with Harvoni (generic) Coverage: AL, AR, CA, CO, DE, GA, HI, ID, IL, IN, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Harvoni (generic) Coverage: AK, AZ, CT, FL, IA, KS, NM, OK, OR, SC, VA, WY
Figure 19. April 2023 Medicaid Coverage - Harvoni (generic)
Map Key: Blue = Harvoni (generic) Coverage; Yellow = No Harvoni (generic) Coverage/No Information regarding Harvoni (generic) Coverage
Epclusa (generic)
States with Epclusa (generic) Coverage: AK, AL, AZ, AR, CA, CO, CT, DE, FL, GA, HI, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Epclusa (generic) Coverage: ID, OK
Figure 20. April 2023 Medicaid Coverage - Epclusa (generic)
Map Key: Blue = Epclusa (generic) Coverage; Yellow = No Epclusa (generic) Coverage/No Information regarding Epclusa (generic) Coverage
April 2023 Notes:
The follow states’ Medicaid programs offer multiple coverage plans for their respective Medicaid clients. The plan highlighted in bold typeface represents the most comprehensive plan with the most drugs covered in the respective state:
Hawaii – (1.) Advantage Plus; (2.) QUEST Integration
New Jersey – (1.) Aetna; (2.) AmeriGroup NJ; (3.) Horizon NJ Health; (4.) UnitedHealthcare of New Jersey; (5.) WellCare
New Mexico – (1.) BlueCross BlueShield of New Mexico; (2.) Presbyterian Centennial Care; (3) Western Sky Community Care
Kentucky has a Unified Medicaid Formulary
Louisiana has a Unified Medicaid Formulary
Ohio – Ohio has a Unified Medicaid Formulary that applies to all MCOs
Oregon’s Medicaid program removed coverage of Sovaldi.
Texas’ Medicaid DPL has not changed, however, the program site notes that Mvyret is now the only preferred DAA, which will no longer require a prior authorization.
New Hampshire’s Medicaid program has removed Zepatier and Sovaldi from the formulary.
Oklahoma’s Medicaid program has reduced coverage to only Mavyret.
Wyoming’s Medicaid program has reduced coverage to only ribavirin products, Mavyret, and Epclusa (GENERIC).
No data is has been made available by the Medicaid programs in the U.S. Territories.
*Medicaid coverage excludes patients from most drug manufacturer patient assistance programs (PAPs)
4. VETERANS PROGRAMS & HCV THERAPIES
The Veteran's Administration (VA) currently offers coverage for all HCV drugs. This is according to the most recent VA National Formulary, dated May 2021 (U.S. Dept. of V.A., 2021a). The VA Treatment Considerations and Choice of Regimen for HCV-Mono-Infected and HIV/HCV Co-Infected Patients, dated March 2021 (U.S. Dept. of V.A., 2021b) lists the following therapies as preferred treatments:
Abbreviations:
- CTP – Child-Turcotte-Pugh (score used to assess severity of cirrhosis)
- IU/mL – International Units Per Milliliter
- PEG-IFN/IFN – Peginterferon/Interferon
- RAS – Resistance-associated substitutions
Genotype 1:
Treatment-naïve without or with cirrhosis (CTP A):
Pangenotypic regimens
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens:
Zepatier: 1 tablet orally daily for 12 weeks if GT1a without baseline NS5A RAS or GT1b
Harvoni: 1 tablet orally daily
If HCV-noninfected, non-cirrhotic, and HCV RNA baseline <6 million IU/mL: 8 weeks
If cirrhotic, baseline HCV RNA ≥6 million IU/mL, HIV/HCV-co-infected, or African American: 12 weeks
Consider adding ribavirin in CTP A patients
Treatment-naïve with decompensated cirrhosis (CTP B or C):
Harvoni: 1 tablet orally daily + ribavirin (600 mg/day and increase by 200 mg/day every 2 weeks only as tolerated) for 12 weeks
Epclusa: 1 tablet orally daily + ribavirin (1000 mg/day - <75kg – or 1,200 mg daily - ≥75kg – orally daily in 2 divided doses with food) for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb).
Treatment-experienced (NS5A- and SOF-naïve [e.g., failed PEG-IFN/RBV ± NS3/4A PI]) without or with cirrhosis (CTP A)
Pangenotypic regimens:
Mavyret: 3 tablets orally daily with food
If PEG-IFN/RBV-experienced: 8 weeks if non-cirrhotic or 12 weeks if cirrhotic
If NS3/4A PI + PEG-IFN/RBV-experienced: 12 weeks
Vosevi: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens
Zepatier: 1 tablet orally daily for 12 weeks if GT1b, or if failed only PEG-IFN/RBV and GT1a without baseline NS5A RAS
Harvoni: 1 tablet orally daily for 12 weeks
Treatment-experienced (NS5A-naïve and SOF-experienced) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food
If PEG-IFN/RBV + Sovaldi-experienced: 8 weeks if non-cirrhotic or 12 weeks if cirrhotic
If Olysio + Sovaldi-experienced: 12 weeks
Epclusa: 1 tablet orally daily for 12 weeks if GT1b
Vosevi: 1 tablet orally daily with food for 12 weeks if GT1a
Treatment-experienced (prior NS5A-containing regimen) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 16 weeks if failed only an NS5A inhibitor without NS3/4A PI (e.g., Harvoni)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-experienced with decompensated cirrhosis (CTP B or C)
Epclusa: 1 tablet orally daily + RBV; start at lower RBV doses as clinically indicated (e.g., baseline Hgb);
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks; NOT FDA approved for 24 weeks
Genotype 2:
Treatment-naïve or treatment-experienced (PEG-IFN/IFN ± RBV or Sovaldi + RBV ± PEG-IFN) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks; 12 weeks if CTP A and treatment-experienced or in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-naïve or treatment-experienced patients with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks
Genotype 3:
Treatment-naïve without cirrhosis or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks if cirrhotic or in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
If CTP A, test for NS5A RAS
Add ribavirin if Y93H RAS present
Treatment-experienced (PEG-IFN ± RBV or Sovaldi + RBV ± PEG-IFN) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 16 weeks
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
If CTP A, consider adding ribavirin (no supporting data)
Treatment-naïve or treatment-experienced with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks
Genotype 4:
Treatment-naïve without or with cirrhosis (CTP A)
Pangenotypic regimens
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens
Zepatier: 1 tablet orally daily for 12 weeks
Harvoni: 1 tablet orally daily for 12 weeks
Treatment-naïve with decompensated cirrhosis (CTP B or C)
Pangenotypic regimen
Epclusa: 1 tablet orally daily + RBV for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
Non-pangenotypic regimen:
Harvoni: 1 tablet orally daily + ribavirin (600 mg/day and increase by 200 mg/day every 2 weeks only as tolerated) for 12 weeks
Treatment-experienced (Sovaldi-experienced and NS5A-naïve) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks if NS3/4A PI-naïve without cirrhosis, and 12 weeks if NS3/4A PI-experienced or CTP A
Epclusa: 1 tablet orally daily + ribavirin for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-experienced with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks; NOT FDA approved for 24 weeks
5. PATIENT ASSISTANCE PROGRAMS
The drug manufacturers and various national nonprofit organizations offer a variation of patient assistance programs (PAPs) to assist patients in accessing treatments. They include:
Support Path (Gilead Sciences):
Financial Assistance
Provides Co-Pay Coupons for Sovaldi, Harvoni, Harvoni (Generic), Epclusa, Epclusa (Generic), and Vosevi
Co-Pay Coupons cover out-of-pocket costs up to 25% of the catalog price of a 12-week regimen (3 bottles/packages) of Sovaldi, Harvoni, Harvoni (Generic), Epclusa, Epclusa (Generic), or Vosevi
Excludes patients enrolled in Medicare Part D or Medicaid
Insurance Support
Researches and verifies patient’s benefits, and gives information they need about coverage options and policies
Explain Prior Authorization process and works with HCV Specialist’s office so they can submit PA forms to a patient’s insurance company
May be able to provide assistance with appeals process
Website: http://www.mysupportpath.com/
AbbVie Mavyret Co-Pay Savings Card:
Financial Assistance
Patient may be eligible to pay as little as $5
Excludes patients enrolled in Medicare Part D, Medicare Advantage, Medigap, Medicaid, TRICARE, Department of Defense, or Veterans Affairs programs)
NeedyMeds:
NeedyMeds Drug Discount Card
Designed to lower cost of prescription medications by up to 80% at participating pharmacies
Price finder tool for the drug discount card
No eligibility requirements
CANNOT be used in combination with government healthcare programs, but CAN be used IN PLACE of program
CANNOT be combined with other offers
Website: http://ow.ly/fEJo309cJ7Z
The Assistance Fund:
Status: WAITLISTED
Requires provider referral
Copay assistance
Eligibility Criteria:
US citizen or permanent resident
Diagnosed with the disease for which you are applying
Prescribed an FDA-approved treatment for the disease
Have prescription coverage for the prescribed treatment
Meet financial eligibility criteria based upon household income and size
Patient Advocate Foundation Co-Pay Relief:
Status: CLOSED
Maximum award of $15,000
Eligibility Requirements:
Patient must be insured, and insurance must cover prescribed medication
Confirmed HCV diagnosis
Reside and receive treatment in the U.S.
Income falls below 400% of FPL with consideration of the Cost of Living Index (COLI) and the number in the household
Patient Access Network (PAN) Foundation:
Status: OPEN
Co-Pay Assistance with a maximum award of $6,000
Patients may apply for a second grant during their eligibility period subject to availability of funding
Eligibility Requirements:
Must be being treated for HCV
Have insurance that covers HCV prescribed medication
Medication must be listed on PAN’s list of covered medications: https://www.panfoundation.org/index.php/en/patients/medications-covered
Income falls below 500% of FPL
Residing and receiving treatment in the U.S. (citizenship NOT required)
Website: https://www.panfoundation.org/index.php/en/patients/assistance-programs/hepatitis-c
HealthWell Foundation:
Status: OPEN
Co-Pay Assistance with a maximum award of $30,000
Minimum Co-Pay Reimbursement Amount: None
Minimum Premium Reimbursement Amount: None
Eligibility Requirements:
Must be being treated for HCV
Have insurance that covers HCV prescribed medication
Income falls below 500% of FPL
Receiving treatment in the U.S.
Website: https://www.healthwellfoundation.org/fund/hepatitis-c/
6. HARM REDUCTION PROGRAMS
Harm Reduction, as it relates to opioid abuse and HCV, are measures designed to serve as preventive or monitoring efforts in combating opioid prescription drug and heroin abuse, and as an effect, helping to prevent the spread of HCV and HIV. The Co-Infection Watch covers the following measures: Syringe Exchange, Expanded Naloxone Access, Good Samaritan Laws, Mandatory PDMP Reporting, Doctor Shopping Laws, Physical Exam Requirements, ID Requirements for Purchase, Required or Recommended Prescriber Education, and Lock-In Programs (Editor’s Note: Program descriptions provided herein).
April 2023 Updates:
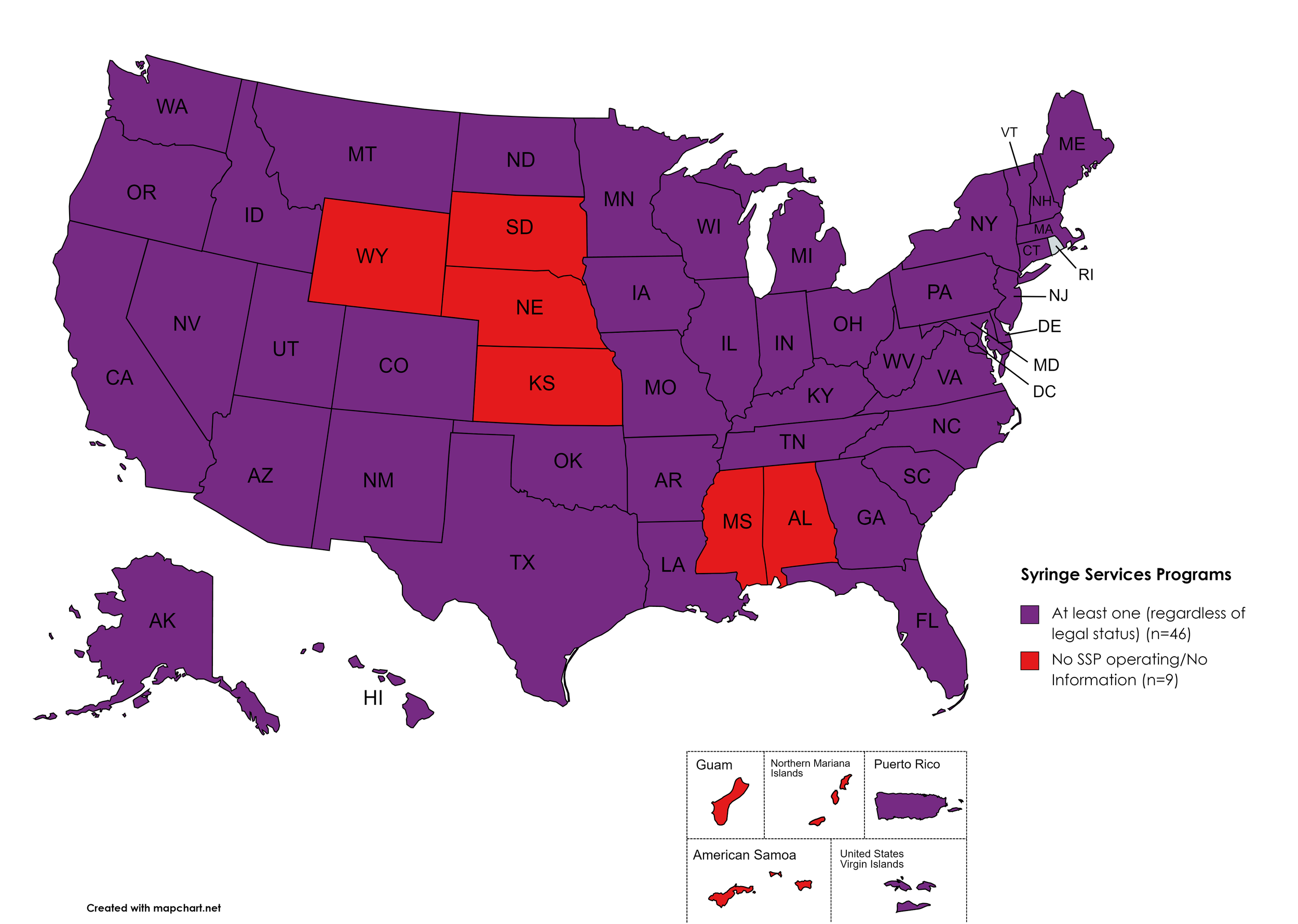
Syringe Exchange
Syringe Services Programs (SSPs) exist to provide injection drug users (or those whose prescriptions require injection) with clean syringes and/or in exchange for used ones. (N.b. – states listed as "at least one SSP…” indicate only that a Syringe Services Program (SSP) exists within the state, regardless of the legality of SSPs under state law).
States with Syringe Exchange: AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MO, MT, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, D.C.
States without Syringe Exchange: AL, KS, MS, NE, SD, WY
Territories with Syringe Exchange: Puerto Rico, U.S. Virgin Islands
Figure 21. April 2023 Syringe Exchange Coverage
Map Key: Purple = Syringe Exchange(s); Red = No Syringe Exchange(s); Grey = No Information
Expanded Naloxone
Naloxone is a drug used to counteract the effects of opioid overdoses. Expanded Access refers to one of more of the following conditions: Naloxone purchase without a prescription; availability to schools, hospitals, and emergency response units for use in the event of an overdose.
States with Expanded Naloxone: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Expanded Naloxone: None
Territories with Expanded Naloxone: Unknown
Figure 22. April 2023 Expanded Naloxone Coverage
Map Key: Purple = Expanded Naloxone; Red = Restricted Naloxone; Gray = No Information
Good Samaritan Laws
Good Samaritan Laws are laws that are designed to protect emergency services personnel, public or private employees, and/or citizens from being held legally liable for any negative healthcare outcomes as a result of providing "reasonable measures" of emergent care.
States with Samaritan Laws: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Samaritan Laws: None
Territories with Samaritan Laws: Unknown
Figure 23. April 2023 Good Samaritan Laws Coverage
Map Key: Purple = Good Samaritan Laws; Red = No Good Samaritan Laws; Gray: No Information
Mandatory PDMP Reporting
Prescription Drug Monitoring Programs (PDMPs) are programs established by state and/or federal law that requires prescribing physicians and the fulfilling pharmacies to report to a state agency one or more of the following data points: Patient Names; Specific Drug(s) Prescribed; Prescription Dosage; Date; Time; Form of State-Issued ID.
States with PDMP Reporting: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without PDMP Reporting: MT, SD
Territories with PDMP Reporting: Guam
Figure 24. April 2023 Mandatory Prescription Drug Monitoring Program Coverage
Map Key: Purple = Mandatory PDMP; Red = No Mandatory PDMP; Gray = No Information
Doctor Shopping Laws
Doctor Shopping Laws are those laws designed to prevent patients from seeking one or more of the same prescription from multiple doctors through the use of subterfuge, falsifying identity, or any other deceptive means. While federal law prohibits Doctor Shopping, most states also include provisions that prohibit patients from seeking a new prescription if another physician has denied a similar prescription within a certain period of time.
States with Doctor Shopping Laws: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Doctor Shopping Laws: None
Territories with Doctor Shopping Laws: None
Figure 25. April 2023 Doctor Shopping Laws Coverage
Map Key: Purple = Doctor Shopping Laws; Red = No Doctor Shopping Laws; Grey = No Information
Physical Exam Required
Physical Exam Requirements are those that mandate that the prescribing physician perform a physical examination on a patient before providing a prescription for a controlled substance to determine if the prescription is medically necessary.
States with Physical Exam Required: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KY, LA, MD, MA, ME, MI, MN, MO, MS, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, PA, RI, SC, TN, TX, UT, VA, VT, WA, WV, WY, D.C.
States without Physical Exam Required: KS, MT, OR, SD, WI
Territories with Physical Exam Required: None
Figure 26. April 2023 Physical Exam Required Coverage
Map Key: Purple = Physical Exam Required; Red: No Physical Exam Required; Grey = No Information
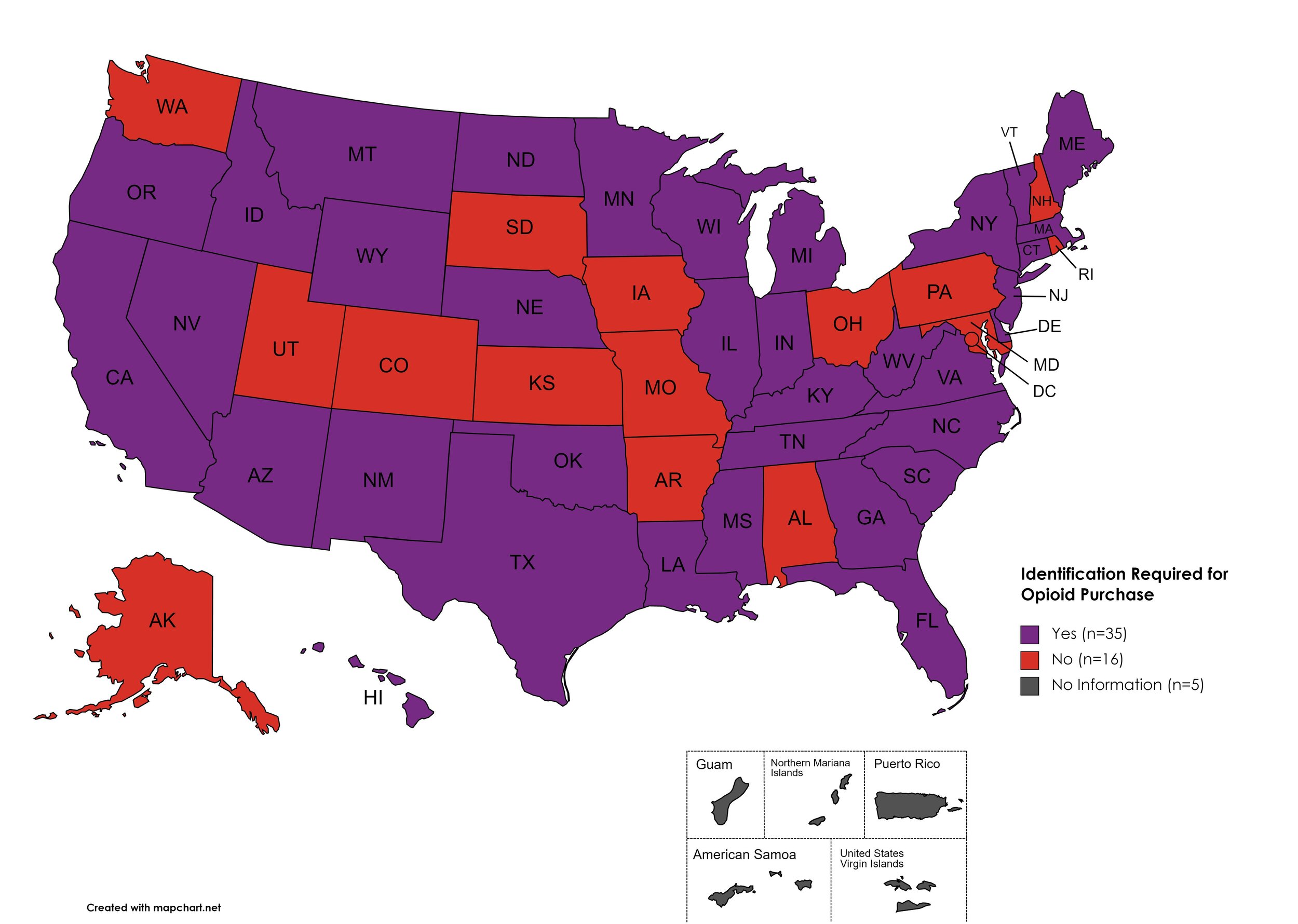
I.D. Required for Purchase of Opioid Prescription
Federal law requires anyone purchase a controlled substance to provide a state-issued identification (“I.D.”) in order to fill the prescription. Mandatory ID requirements go further and require that this information be recorded and stored in an effort to prevent the same patient from obtaining multiple or repeated prescriptions in a given period of time.
States with I.D. Required: AZ, CA, CT, DE, FL, GA, HI, ID, IL, IN, KY, LA, ME, MA, MI, MS, MN, MT, NE, NV, NJ, NM, NY, NC, ND, OK, OR, SC, TN, TX, VT, VA, WV, WI, WY
States without I.D. Required: AL, AK, AR, CO, IA, KS, MD, MO, NH, OH, PA, RI, SD, UT, WA, D.C.
Territories with I.D. Required: Unknown
Figure 27. April 2023 I.D. Required Coverage
Map Key: Purple = I.D. Required; Red = No I.D. Required; Gray = No Information
Prescriber Education Required/Recommended
States that require/do not require that prescribing physicians undergo special training related to safer prescribing and utilization practices.
States with Prescriber Education Required: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MS, NE, NV, NH, NJ, NM, NY, NC, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Prescriber Education Required: KS, MO, MT, ND, SD
Territories with Prescriber Education Required: Unknown
Figure 28. April 2023 Prescriber Education Required Coverage
Map Key: Purple = Prescriber Ed Required; Red = No Prescriber Ed Required; Gray = No Information
Medicaid Lock-In Program
Lock-In Programs are laws requiring that patients either receive prescriptions from only one physician and/or fill prescriptions from only one pharmacy.
States with Medicaid Lock-In Program: AL, AK, AZ, AR, CA, CO, CT, DE, GA, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Medicaid Lock-In Program: FL, HI, SD
Territories with Medicaid Lock-In Program: Unknown
Figure 29. April 2023 Medicaid Lock-In Coverage
Map Key: Purple = Medicaid Lock-In; Red = No Medicaid Lock-In; Gray = No Information
April 2023 Notes:
MO has begun implementation of PDMP reporting. Legislation was passed in 2021, however the statewide program did not begin implementation immediately.
CANN is no longer able to independently verify the existence of an SSP in Kansas. KS state laws prohibit SSPs and syringes are included in the state’s drug paraphernalia law.
The DEA’s COVID-19-based waiver of in-person exams is expected to expire in November of 2023. A final rule on this change has not yet been published as of the time of this report.
While several states are proposing expansions of various monitored Harm reduction metrics, these metrics will be reviewed in the July 2023 HIV-HCV Co-Infection Watch Report, wherein the majority of states will have either passed or not passed laws affecting this section.
7. COVID-19 IMPACT ON HIV & HCV
The Community Access National Network’s blog began 2021 by assessing COVID-19’s impact on HIV, HCV, and Substance-Use Disorder. We've subsequently followed-up by asking, COVID-19: How Far We’ve Come & How Far We Have to Go? We continue to monitor developments in light of the ongoing COVID-19 pandemic and its impacts on public health.
Additional Resources and Relevant Issues:
Biden to End Covid Health Emergency - In January 2023, the Biden Administration announced it would renew the COVID-19 declaration of national public health emergency in May 2023, after one final renewal.
In March, the Senate passed an already House approved resolution to end the COVID-19 public health emergency early. President Biden did not veto the measure, bringing an end to the COVID-19 3-yeard old public health emergency. This is slightly earlier than the timeline President Biden had promised state public health entities in terms of notice.
For more on the “unwinding” of the public health emergency and what it means for patients and public health entities, check out Kaiser Family Foundation’s (KFF) primer. KFF has been monitoring and updating state policy actions and estimations on impact.
Wave of Departing Public Health Workers Puts America at Risk - Governing.com reviews the the concern with public health capacity by focusing on the single, most important resource public health holds: people. Public health programs and their successes are largely dependent upon a sense of person investment and community knowledge, which cannot be taught in text books. The COVID-19 pandemic and resulting backlash - both socially and politically, as dollars have been drawn down and budgets left to atrophy - has left the field expecting to see a dramatic decrease in manpower in the coming few years. While technological advancements and streamlining processes may help stem some of that negative potential impacts, they cannot compensate completely.
Health Affairs Study: Exodus of State and Local Public Health Employees - Health Affairs reviewed separations before and throughout the COVID-19 public health emergency. On top of losing manpower to perform basic tasks, institutions are expected to lose institutional knowledge, with the majority of employees having 5 or fewer years of experience. At the same time, the highest rate of turnover in public health agencies is expected in the under 35 crowd. Chief reasons among surveyed participants, a lack of benefits equitable to private sector work, a sense of job stability, and a lack of satisfaction with supervisors ranked high on why people were anticipating leaving public health. Lack of state investment into these spaces is pushing this critical government function into further uncertainty - fait accompli as it were.
Opinion: The Next Public Health Crisis will be Understaffed Local Health Departments - Guest contributor, Sarah Ravenhall of the New York State Association of County Healthy Officials, opines that losing public health expertise and manpower is a health crisis waiting to happen - in and of itself. “If we ignore this crisis, our public health system will crumble, inviting disease, death, higher costs and chaos. We must act now, and resolutely.”
White House Launching $5B Program to Speed Coronavirus Vaccines - Despite the end of the public health emergency, the sense of emergency and potential for COVID-19 to mutate again or another coronavirus to cross-over into the human population with devastating effects remains an issue of concern. The Biden Administration is proposing a plan that would encourage expedited development of a COVID-19 vaccine that would address the universe of variants and potentially be easier to administer, with a special focus on nasal vaccine development.
8. LATEST NEWS
****IMPORTANT**** Braidwood Decision - On March 30th, Judge Reed O’Connor issued a judgment in the case concerning the United States Preventative Services Task Force and, more directly, the requirement of Affordable Care Act (ACA)affected health plans mandate to cover pre-exposure prophylaxis for the prevention of HIV. The case has reverberated around the HIV advocacy community and has massive potential impacts among a plethora of preventative care services across the country. The Biden Administration is expected to seek a stay of the ruling while seeking appeal to the 5th Circuit. O’Connor’s previous ACA-related rulings have been overturned when they reach the United States Supreme Court, which could still be more than a year away as the case makes its way through the court system.
****IMPORTANT**** FDA Approval and Authorities - In competing rulings issued Friday, April 7th, the U.S. Food and Drug Administration (FDA) was ordered to keep the medication mifepristone available while simultaneously having the 20-year approval “stayed” in another court. The FDA has already appealed the “stay” decision to the 5th Circuit and Attorneys General for plaintiff states in the other decision has asked for clarity due to the competing rulings. The “stay” ruling is particularly concerning because it undermines the authority of the FDA to approve the safety of medications, specifically those afforded “accelerated approval” under 21 CFR Part 314 subpart H, a designation which has been largely used by manufacturers bringing antiretroviral medications to market.
Renewal of Determination That a Public Health Emergency Exists - On March 31, 2023, the U.S. Department of Health and Human Services (HHS) Secretary Xavier Becerra renewed the ongoing declaration of public health emergency regarding the opioid crisis. The declaration is the latest in extensions regarding the opioid crisis, originating in October 2017.
The Lonely Work of HIV Harm Reduction in Prison Filter contributor Jonathan Kirkpatrick explores his experiences as an incarcerated person living with HIV and navigating HIV and Hepatitis C education and health care access from within a prison system. Kirkpatrick has previously written about how he was placed in solitary confinement due to his HIV diagnosis. Detailing how some incarcerated persons discover their HIV status after being screened during intake processes, requirements an incarcerated person file a formal assault report in order to access post-exposure prophylaxis (which comes with the risk of retaliation and not timely receiving the medication), and how accessing harm reduction materials like condoms and PrEP are impossible because - legally - incarcerated persons cannot consent to sex. Similarly, syringe services are not made available in prisons, despite a need for same. Given the prevalence of HIV and Hepatitis C among incarcerated and previously-incarcerated people, this area presents an important opportunity of advocacy.
PEPFAR Turns 20 with More than 25 Million Lives Saved - As the President’s Emergency Plan for AIDS Relief (PEPFAR) turns 20, the global program focused on supporting HIV service organizations abroad and providing life-saving medications is up for reauthorization in a polarized Congress. In the past, the program has received wide bipartisan support. PEPFAR remains one of President George W. Bush’s lasting legacy items, serving the needs of millions worldwide, having increased the number of people accessing antiretroviral medications by more than 300 times since inception.
HIV/AIDS Funding took Backseat During COVID-19 Pandemic - Experts in Canada and abroad discuss how global HIV funding shifted out of focus for many governments across the globe during the COVID-19 pandemic. Research in Canada, as much of the rest of the world, is focused on hunting out viral reservoirs, because effective antiretroviral treatment already exists. Officials and advocates across Canada share a concern over decreased testing and linkage to care during the pandemic and don’t believe the primary barrier to ending the HIV Epidemic is science, but political will and investment.
Oregon’s HIV Alliance Co-Hosts Substance Use Disorder Recovery Discussion and Resource Fair - In April 2023, a group of hospital, community health center, public health officials, and more hosted a symposium seeking to educate attendees, including professionals, about substance use disorder (SUD), address stigma associated with addiction and recovery, as well as about resources made available in the community regarding these and subsequent concerns around infectious diseases like HIV, STIs, and HCV. The HIV Alliance sees this event as a necessity in curbing new HIV diagnoses and overdose deaths.
Population-Level Health Effects of Involuntary Displacement of People Experiencing Unsheltered Homelessness Who Inject Drugs in US Cities - As cities employ a variety of tactics to address unhoused populations, sharp focus has come to tactics which involve police raids or “sweeps” of encampments. One lacking data point, however, is what role or impact this action plays under a public health lens. In an original investigation, authors use a closed cohort microsimulation model to estimate overdose mortality, serious injection-related infections and mortality related to serious injection-related infections, hospitalizations, initiations of medications for opioid use disorder, and life-years lived over a 10-year period for two scenarios: “no displacement” and “continual involuntary displacement”. The models’ results are dramatic increases in mortality for both overdose and injection-related infections and hospitalizations while dramatically decreasing likelihood of initiating medication assisted treatment for the “continual involuntary displacement” cohort. This study adds data to a nation of addressing houselessness as a public health concern, rather than a crime-related one.
Naloxone Expansion is NOT Associated with Increases in Adolescent Heroin Use and Injection Drug Use - A common retort to expanding effort to increase access to the overdose reversal medication Naloxone often cite a moralized view that saving those lives only sets them up to repeat the offending behavior - or the idea of risk mitigation, wherein because a factor of access reduces the risk of a fatal overdose, people who use drugs will increase their risk behaviors. However, data from 44 States proves this theory wrong, giving support to fight theories of “social contagion” with regard to medicalized overdose interventions. Results of the review found Naloxone access and pharmacy distribution were more consistently associated with DECREASES in lifetime heroin and injection drug use among adolescents. Specifically, because of the reviews’ findings, authors urge policymakers to further improve and expand adolescent access to Naloxone.
Why are We Denying People with opioid Addiction the Most Effective Treatment? Guest contributor to the Los Angeles Times, Dr. Ashish Thakrar, an internal medicine and addiction treatment physician, asks “If we can expand access to naloxone, why can’t we expand treatment to medication assisted treatment for opioid use disorder?” Dr. Thakrar shares a frustration with federal funding and regulatory requirements which force those seeking medication assisted treatment to visit “opioid treatment programs” (OTPs), especially given less than 2,000 OTPs existed in 2018 and less than 80% of counties across the country have an OTP. Indeed, with the DEA’s COVID-waiver rules coming to an end, the reprieve of being able to access medications for the treatment of opioid use disorder could very quickly be ripped away from patients who, from 2020 through November of this year, had ready access to telehealth options for initiating and maintaining their medications and recovery programs. One solution, Dr. Ashkrar argues, can be found in the Modernizing Opioid Treatment Access Act, introduced to the Senate in March. Dr. Ashkrar further argues that federal agencies should move expeditiously to ensure patients retain and gain ready access to medication assisted treatment programs by reducing a variety of barriers to care, including in-person prescribing and administration requirements, among others.