Watch 01: January 2023
The HIV/HCV Co-Infection Watch is a project of the Community Access National Network (CANN) designed to research, monitor and report on HIV and Hepatitis C (HCV) co-infection in the United States. The January 2023 Watch includes timely updates herein. To read the project disclaimer and/or methodology, CLICK HERE.
1. FINDINGS
The following is a summary of the key findings for January 2023:
AIDS Drug Assistance Programs:
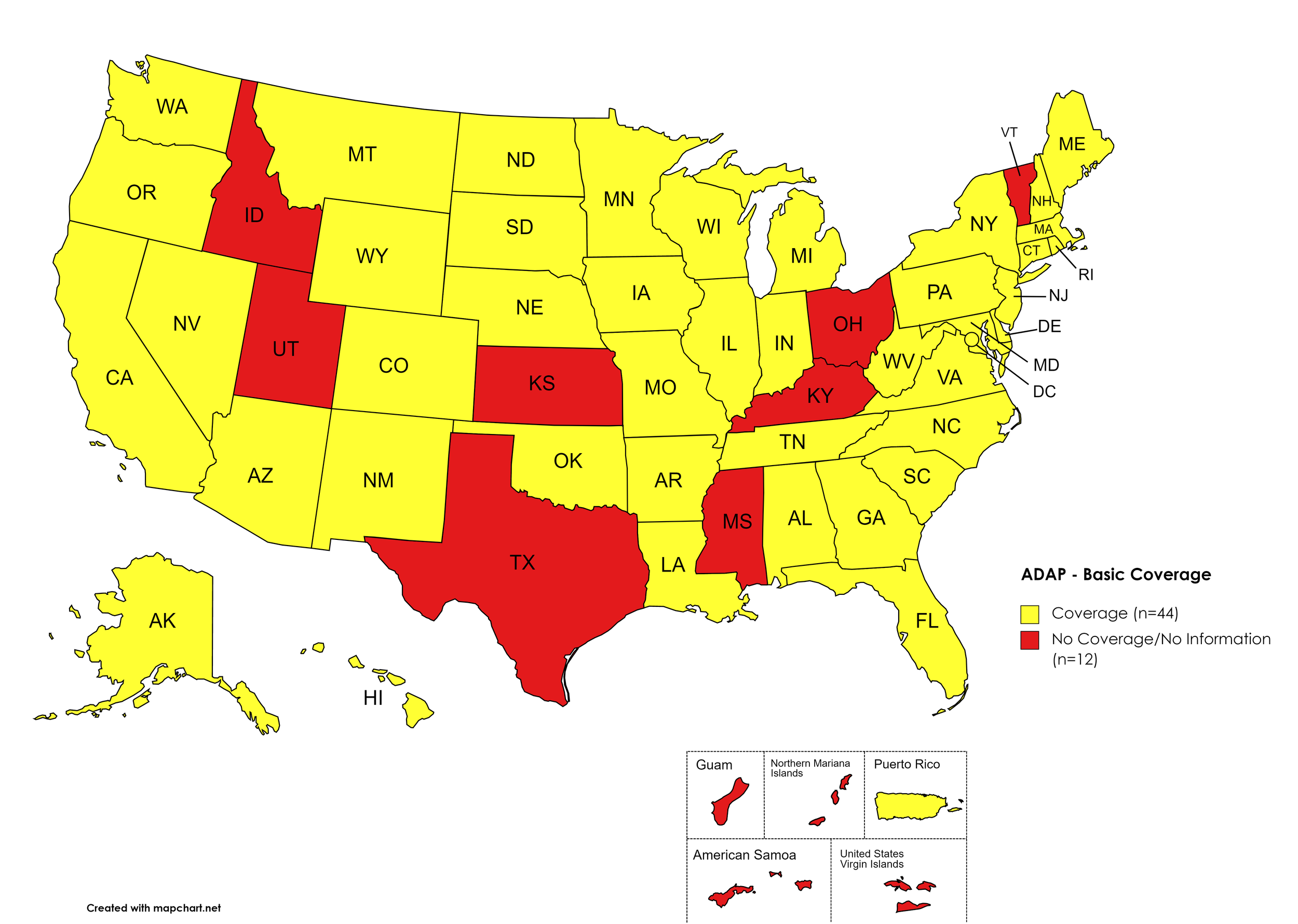
There are 56 State and Territorial AIDS Drug Assistance Programs (ADAPs) in the United States, 48 of which offer some form of coverage for Hepatitis C (HCV) treatment. Of those programs, 46 have expanded their HCV coverage to include the Direct-Acting Antiviral (DAA) regimens that serve as the current Standard of Care (SOC) for Hepatitis C treatment. Two (2) programs offer only Basic Coverage and 8 programs offer No Coverage. Two (2) programs cover only a single Direct-Acting Antiviral. Three (3) territories – American Samoa, Marshall Islands, and Northern Mariana Islands – are not accounted for in this data. A state-by-state Drug Formulary breakdown of coverage is included in the January 2023 Updates, with accompanying drug-specific maps in Figures 1 – 10.
Medicaid Programs:
There are 59 State and Territorial Medicaid programs in the United States, and data is represented for all fifty (50) states and the District of Columbia. As of October 01, 2016, all 50 states and the District of Columbia offer Expanded Coverage. A state-by-state PDL breakdown of coverage is included in the January 2023 Updates, with accompanying drug-specific maps in Figures 11 – 20.
Harm Reduction Programs:
Every State and Territory in the United States currently provides funding for low-income people living with substance abuse issues to enter state-funded rehabilitation services (National Center for Biotechnology Information, n.d.). Forty-four (44) States, the District of Columbia and three (3) Territories currently have Syringe Services Programs (SSPs) in place, regardless of the legality. Fifty (50) States and the District of Columbia have expanded access to Naloxone to avert opioid drug overdoses. Fifty (50) States and the District of Columbia have Good Samaritan laws or statutes that provide some level of protection for those rendering emergency services during drug overdoses. Forty-seven (47) States, the District of Columbia, and Guam make reporting to Prescription Drug Monitoring Programs (PDMPs) mandatory, requiring physicians and/or pharmacists to report prescriptions written or filled to a state agency for monitoring. Fifty (50) States and the District of Columbia have Opioid-Specific Doctor Shopping Laws preventing patients from attempting to receive multiple prescriptions from numerous physicians, and/or from withholding information in order to receive prescriptions. Forty-five (45) states and the District of Columbia mandate a Physical Exam Requirement in order for patients to receive a prescription for opioid drugs. Thirty-Five (35) states have in place an ID Requirement mandating that people filling opioid prescriptions present a state-issued ID prior to receiving their prescription. Forty-five (45) states and the District of Columbia require prescribing physicians to attend mandatory and continuing opioid prescribing education sessions. Forty-seven (47) states and the District of Columbia have Medicaid doctor/pharmacy Lock-In programs that require patients to receive prescriptions from a single physician and/or fill prescriptions from a single pharmacy. A state-by-state program breakdown is included in the January 2023 Updates, with accompanying drug-specific maps in Figures 21-29.
2. AIDS DRUG ASSISTANCE PROGRAMS (ADAPs) & HCV THERAPIES
Of the 56 respective State and Territorial ADAPs, only 8 (KS, KY, OH, UT, VT, GU, PW, VI) do not offer any coverage for HCV drug therapies. States whose formularies are not available on the state-run website have been checked against the most recent National Alliance of State and Territorial AIDS Directors (NASTAD) formulary database (last updated January 1, 2022). The data presented are current as of January 20, 2023.
January 2023 Updates:
Basic Coverage
States with Basic HCV Medications Coverage: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, IL, IN, IA, LA, ME, MD, MA, MI, MN, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OK, OR, PA, RI, SC, SD, TN, VA, WA, WV, WI, WY, D.C.
States without Basic HCV Medications Coverage: ID, KS, KY, MS, OH, TX, UT, VT
Territories with Basic HCV Medications Coverage: P.R.
Figure 1. January 2023 ADAP Coverage - Basic HCV Medications
Map Key: Yellow = Basic HCV Medication Coverage; Red = No Basic HCV Medication Coverage/No Information regarding Basic HCV Medication Coverage
Sovaldi
States with Sovaldi Coverage: AZ, CA, CO, GA, HI, IL, IN, IA, LA, ME, MD, MA, MN, NE, NV, NH, NJ, NM, ND, OK, OR, PA, SD, VA, WA, WI, WY, D.C.
States without Sovaldi Coverage: AL, AK, AR, CT, DE, FL, ID, KS, KY, MI, MS, MO, MT, NY, NC, OH, RI, SC, TN, TX, UT, VT, WV
Territories with Sovaldi Coverage: P.R.
Figure 2. January 2023 ADAP Coverage - Sovaldi
Map Key: Yellow = Sovaldi Coverage; Red = No Sovaldi Coverage/No Information regarding Sovaldi Coverage
Harvoni
States with Harvoni Coverage: AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, NE, NV, NH, NJ, NM, NC, ND, OK, OR, PA, SD, TN, VA, WA, WI, WY, D.C.
States without Harvoni Coverage: AL, AK, KS, KY, MO, MT, NY, OH, RI, SC, TX, UT, VT, WV
Territories with Harvoni Coverage: P.R.
Figure 3. January 2023 ADAP Coverage - Harvoni
Map Key: Yellow = Harvoni Coverage; Red = No Harvoni Coverage/No Information regarding Harvoni Coverage
Zepatier
States with Zepatier Coverage: AL, AZ, AR, CA, CO, FL, GA, HI, IL, IA, LA, ME, MD, MA, MI, MN, MS, NE, NV, NH, NJ, NM, NY, NC, ND, OR, PA, SD, VA, WA, WV, WI, WY, D.C.
States without Zepatier Coverage: AK, CT, DE, ID, IN, KS, KY, MO, MT, OH, OK, RI, SC, TN, TX, UT, VT
Territories with Zepatier Coverage: P.R.
Figure 4. January 2023 ADAP Coverage - Zepatier
Map Key: Yellow = Zepatier Coverage; Red = No Zepatier Coverage/No Information regarding Zepatier Coverage
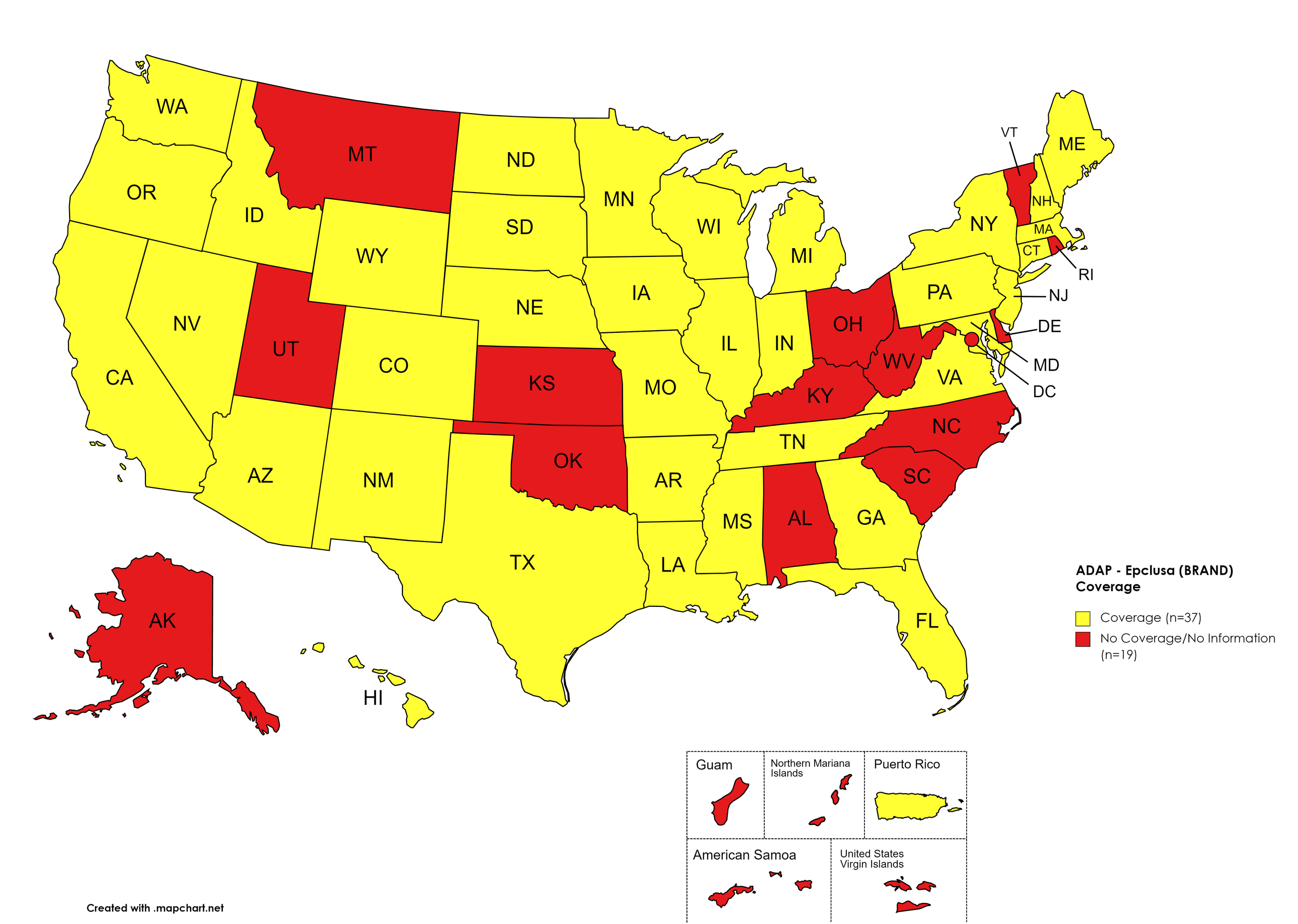
Epclusa
States with Epclusa Coverage: AZ, AR, CA, CO, CT, FL, GA, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, MO, NE, NY, NV, NH, NJ, NM, ND, OR, PA, SD, TN, TX, VA, WA, WI, WY
States without Epclusa Coverage: AL, AK, DE, KS, KY, MT, NC, OH, OK, RI, SC, UT, VT, WV, D.C.
Territories with Epclusa Coverage: P.R.
Figure 5. January 2023 ADAP Coverage - Epclusa
Map Key: Yellow = Epclusa Coverage; Red = No Epclusa Coverage/No Information regarding Epclusa Coverage
Vosevi
States with Vosevi Coverage: CA, CT, FL, HI, ID, IL, IN, IA, LA, MD, MA, MN, NE, NV, NH, NJ, NM, ND, OR, SD, TN, WA, WY
States without Vosevi Coverage: AL, AK, AZ, AR, CO, DE, GA, KS, KY, ME, MI, MS, MO, MT, NY, NC, OH, OK, PA, RI, SC, TX, UT, VT, VA, WV, WI, D.C.
Territories with Vosevi Coverage: P.R.
Figure 6. January 2023 ADAP Coverage - Vosevi
Map Key: Yellow = Vosevi Coverage; Red = No Vosevi Coverage/No Information regarding Vosevi Coverage
Mavyret
States with Mavyret Coverage: AL, AZ, AR, CA, CO, CT, FL, GA, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OR, PA, SD, TN, VA, WA, WV, WI, WY, D.C.
States without Mavyret Coverage: AK, DE, KS, KY, OH, OK, RI, SC, TX, UT, VT
Territories with Mavyret Coverage: P.R.
Figure 7. January 2023 ADAP Coverage - Mavyret
Map Key: Yellow = Mavyret Coverage; Red = No Mavyret Coverage/No Information regarding Mavyret Coverage
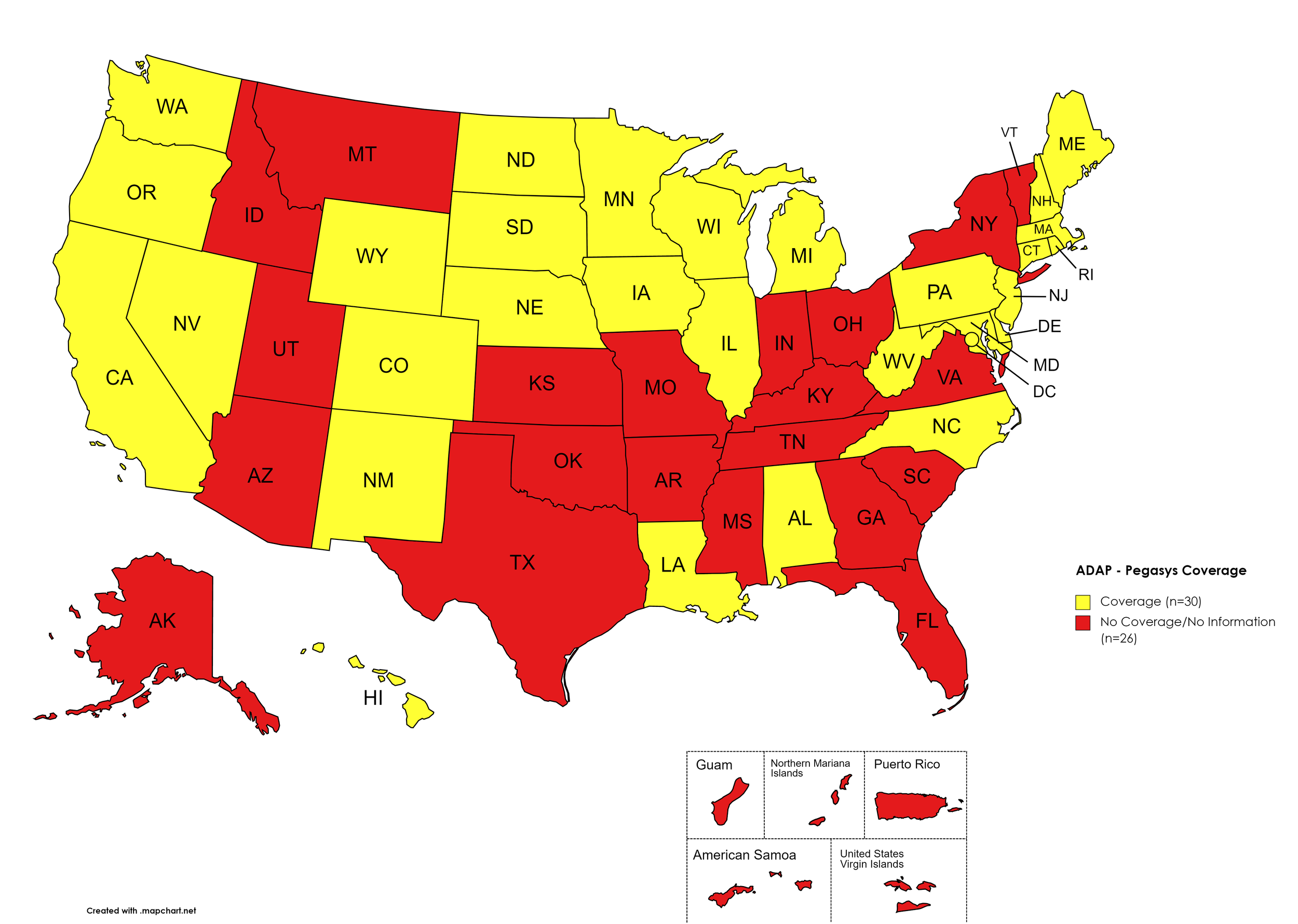
Pegasys
States with Pegasys Coverage: AL, CA, CO, CT, DE, HI, IL, IA, LA, ME, MD, MA, MI, MN, NE, NV, NH, NJ, NM, NC, ND, OR, PA, RI, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Pegasys Coverage: AK, AZ, AR, FL, GA, ID, IN, KS, KY, MS, MO, MT, NY, OH, OK, SC, TN, TX, UT, VT, VA
Territories with Pegasys Coverage: None/Unknown
Figure 8. January 2023 ADAP Coverage - Pegasys
Map Key: Yellow = Pegasys Coverage; Red = No Pegasys Coverage/No Information regarding Pegasys Coverage
Harvoni (generic)
States with Harvoni (generic) Coverage: AZ, AR, CA, CO, CT, FL, IL, IA, ME, MD, MA, MN, MS, NE, NV, NH, NJ, NM, NC, ND, OK, OR, PA, SD, TN, WA, WI, WY, D.C.
States without Harvoni (generic)Coverage: AL, AK, DE, GA, HI, ID, IN, KS, KY, LA, MI, MO, MT, NY, OH, RI, SC, TX, UT, VT, VA, WV
Territories with Harvoni (generic) Coverage: P.R.
Figure 9. January 2023 ADAP Coverage - Harvoni (Generic)
Map Key: Yellow = Harvoni (Generic) Coverage; Red = No Harvoni (Generic) Coverage/No Information regarding Harvoni (Generic) Coverage
Epclusa (generic)
States with Epclusa (generic) Coverage: AZ, AR, CA, CO, CT, FL, IL, IN, IA, ME, MD, MA, MN, MS, MO, NE, NV, NH, NJ, NM, ND, OR, PA, SD, TN, WA, WI, WY, D.C.
States without Epclusa (generic) Coverage: AL, AK, DE, GA, HI, ID, KS, KY, LA, MI, MT, NY, NC, OH, OK, RI, SC, TX, UT, VT, VA, WV
Territories with Epclusa (generic) Coverage: P.R.
Figure 10. January 2023 ADAP Coverage - Epclusa (generic)
Map Key: Yellow = Epclusa (generic) Coverage; Red = No Epclusa (generic) Coverage/No Information regarding Epclusa (generic) Coverage
January 2023 Notes:
States with Open Formularies: IL, IA, MA, MN, NE, NH, NJ, NM, ND, OH, OR, WA, WY
N.B. – Although Ohio is listed by NASTAD as having an open formulary, both NASTAD’s ADAP Formulary Database and Ohio’s ADAP website indicates that the state does not offer any treatment for HCV.
N.B. – Although North Dakota has adopted an open formulary, they provide only co-pay and deductible assistance for HCV medications.
N.B. – Wyoming's ADAP Open Formulary document, the following disclaimer related to HCV is made: Hepatitis C treatment medications (i.e. Harvoni, Sovaldi, Ribavirin, Zepatier, Epclusa) must be prior authorized. To be eligible, clients must have applied for prior authorization from their insurance plan and the WY ADAP Hepatitis C Treatment checklist must be completed and signed by the provider and client.
Colorado offers five coverage options – Standard ADAP, HIV Medical Assistance Program (HMAP), Bridging the Gap Colorado (BTGC), HIV Insurance Assistance Program (HIAP), and Supplemental Wrap Around Program (SWAP). ‘Yes’ indications in Figure 1. for Colorado denote that at least one of these programs offers coverage for each respective drug. The Standard ADAP Formulary covers medications only if funds are available to do so.
Louisiana’s ADAP (Louisiana Health Access Program – LA HAP) offers two coverage options – Uninsured (Louisiana Drug Assistance Program – L-DAP) and Insured (Health Insurance Program – HIP). HIP pays for the cost of treatment only if the client’s primary insurance covers the drug under its formulary.
Georgia’s ADAP notes the following: “Georgia ADAP Hepatitis C Program is currently on HOLD until future funding is available. Please utilize Patient Assistance Programs (PAP’s) for Hepatitis C medications.”
Texas ADAP’s coverage of HCV medications is limited to Epclusa (brand).
3. MEDICAID PROGRAMS & HCV THERAPIES
All 50 states and the District of Columbia continue to offer some form of HCV coverage. All 50 states and the District of Columbia have expanded their Preferred Drug Lists to include at least one HCV Direct Acting Agent (DAA).
January 2023 Updates:
Basic Coverage
States with Basic HCV Medications Coverage: AZ, AK, AR, CA, CO, CT, DE, FL, GA, HI, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OR, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Basic HCV Medications Coverage: AL, ID, KS, MO, OK, SC, VA, WY
Figure 11. January 2023 Medicaid Coverage - Basic HCV Medications
Map Key: Blue = Basic HCV Medication Coverage; Yellow = No Basic HCV Medication Coverage/No Information regarding Basic HCV Medication Coverage
Sovaldi
States with Sovaldi Coverage: AR, CA, CO, DE, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NY, NC, ND, OH, OK, PA, RI, SD, TN, TX, UT, VT, WA, WI, WY, D.C.
States without Sovaldi Coverage: AL, AK, AZ, CT, FL, IA, NM, OR, SC, VA, WV
Figure 12. January 2023 Medicaid Coverage - Sovaldi
Map Key: Blue = Sovaldi Coverage; Yellow = No Sovaldi Coverage/No Information regarding Sovaldi Coverage
Harvoni
States with Harvoni Coverage: AL, AR, CA, CO, DE, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NY, NC, ND, OH, OK, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, WY, D.C.
States without Harvoni Coverage: AK, AZ, CT, FL, IA, NM, OR, SC, VA
Figure 13. January 2023 Medicaid Coverage - Harvoni
Map Key: Blue = Harvoni Coverage; Yellow = No Harvoni Coverage/No Information regarding Harvoni Coverage
Zepatier
States with Zepatier Coverage: AL, AR, CA, CO, DE, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WI, WY, D.C.
States without Zepatier Coverage: AK, AZ, CT, FL, IA, NM, OK, OR, SC, VA, WV
Figure 14. January 2023 Medicaid Coverage - Zepatier
Map Key: Blue = Zepatier Coverage; Yellow = No Zepatier Coverage/No Information regarding Zepatier Coverage
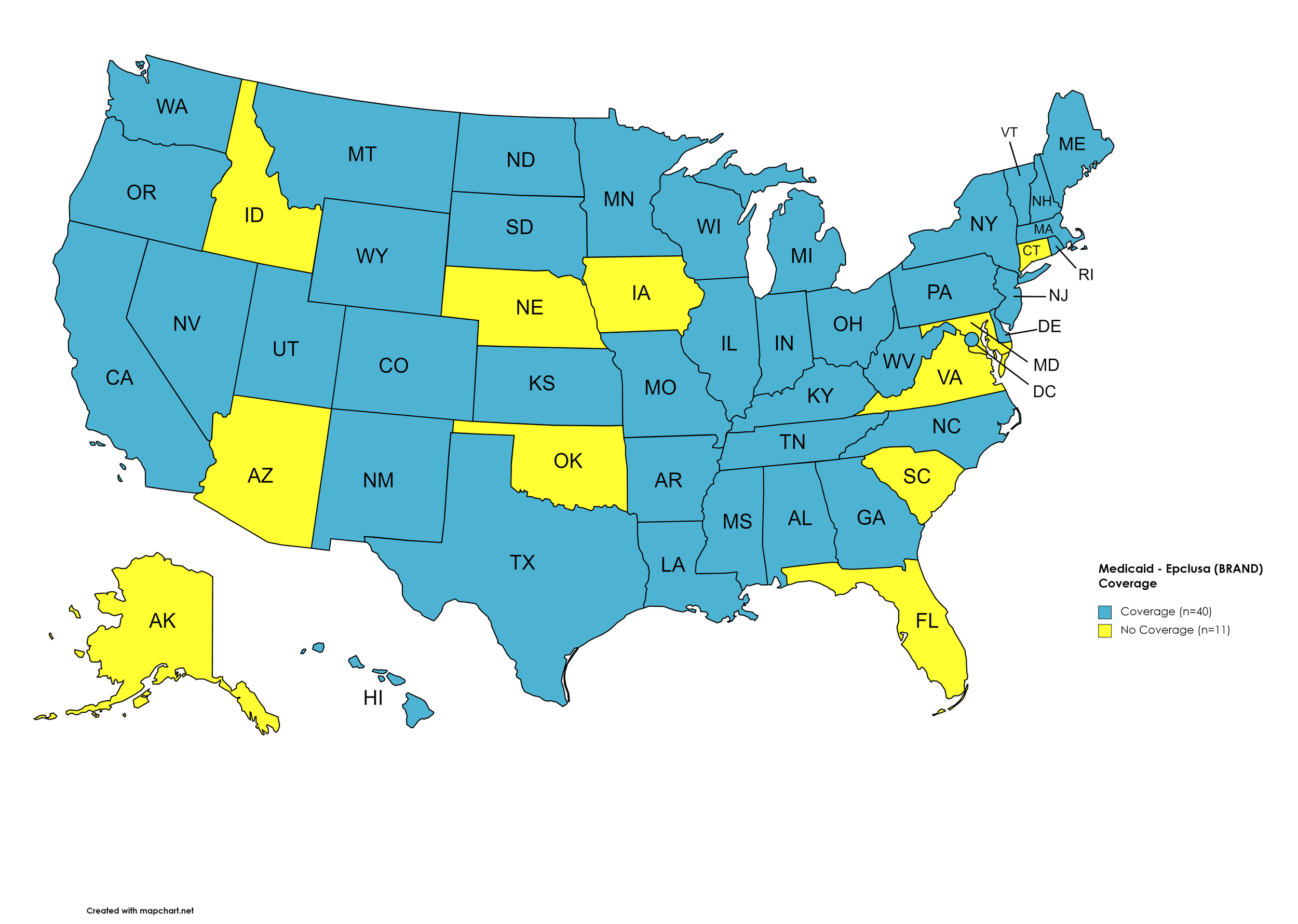
Epclusa
States with Epclusa Coverage: AL, AR, CA, CO, DE, GA, HI, IL, IN, KS, KY, LA, MA, ME, MI, MN, MS, MO, MT, NV, NH, NJ, NM, NY, NC, ND, OH, OR, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, WY, D.C.
States without Epclusa Coverage: AK, AZ, CT, FL, ID, IA, MD, NE, OK, SC, VA
Figure 15. January 2023 Medicaid Coverage - Epclusa
Map Key: Blue = Epclusa Coverage; Yellow = No Epclusa Coverage/No Information regarding Epclusa Coverage
Vosevi
States with Vosevi Coverage: AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NY, NC, ND, OH, PA, RI, SC, SD, TN, TX, UT, VT, WA, WI, WY, D.C.
States without Vosevi Coverage: AL, AK, AZ, NM, OK, OR, VA, WV
Figure 16. January 2023 Medicaid Coverage - Vosevi
Map Key: Blue = Vosevi Coverage; Yellow = No Vosevi Coverage/No Information regarding Vosevi Coverage
Mavyret
States with Mavyret Coverage: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
Figure 17. January 2023 Medicaid Coverage - Mavyret
Map Key: Blue = Mavyret Coverage; Yellow = No Mavyret Coverage/No Information regarding Mavyret Coverage
Pegasys
States with Pegasys Coverage: AK, AZ, CA, CT DE, FL, GA, HI, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MS, MT, NE, NV, NH, NJ, NM, NY, NC, OH, OR, PA, RI, SD, TN, TX, VT, WA, WV, WI, D.C.
States without Pegasys Coverage: AL, AR, CO, ID, KS, MO, ND, OK, SC, UT, VA, WY
Figure 18. January 2023 Medicaid Coverage - Pegasys
Map Key: Blue = Pegasys Coverage; Yellow = No Pegasys Coverage/No Information regarding Pegasys Coverage
Harvoni (generic)
States with Harvoni (generic) Coverage: AL, AR, CA, CO, DE, GA, HI, ID, IL, IN, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NY, NC, ND, OH, OK, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Harvoni (generic) Coverage: AK, AZ, CT, FL, IA, KS, NM, OR, SC, VA, WY
Figure 19. January 2023 Medicaid Coverage - Harvoni (generic)
Map Key: Blue = Harvoni (generic) Coverage; Yellow = No Harvoni (generic) Coverage/No Information regarding Harvoni (generic) Coverage
Epclusa (generic)
States with Epclusa (generic) Coverage: AK, AL, AZ, AR, CA, CO, CT, DE, FL, GA, HI, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Epclusa (generic) Coverage: ID, OK
Figure 20. January 2023 Medicaid Coverage - Epclusa (generic)
Map Key: Blue = Epclusa (generic) Coverage; Yellow = No Epclusa (generic) Coverage/No Information regarding Epclusa (generic) Coverage
January 2023 Notes:
The follow states’ Medicaid programs offer multiple coverage plans for their respective Medicaid clients. The plan highlighted in bold typeface represents the most comprehensive plan with the most drugs covered in the respective state:
Hawaii – (1.) Advantage Plus; (2.) QUEST Integration
New Jersey – (1.) Aetna; (2.) AmeriGroup NJ; (3.) Horizon NJ Health; (4.) UnitedHealthcare of New Jersey; (5.) WellCare
New Mexico – (1.) BlueCross BlueShield of New Mexico; (2.) Presbyterian Centennial Care; (3) Western Sky Community Care
Kentucky has a Unified Medicaid Formulary
Louisiana has a Unified Medicaid Formulary
Ohio – Ohio has a Unified Medicaid Formulary that applies to all MCOs
Oregon’s Medicaid program removed coverage of Sovaldi.
Texas’ Medicaid DPL has not changed, however, the program site notes that Mvyret is now the only preferred DAA, which will no longer require a prior authorization.
West Virginia’s Medicaid program removed coverage of Sovaldi, Zepatier, and Vosevi.
No data is has been made available by the Medicaid programs in the U.S. Territories.
*Medicaid coverage excludes patients from most drug manufacturer patient assistance programs (PAPs)
4. VETERANS PROGRAMS & HCV THERAPIES
The Veteran's Administration (VA) currently offers coverage for all HCV drugs. This is according to the most recent VA National Formulary, dated May 2021 (U.S. Dept. of V.A., 2021a). The VA Treatment Considerations and Choice of Regimen for HCV-Mono-Infected and HIV/HCV Co-Infected Patients, dated March 2021 (U.S. Dept. of V.A., 2021b) lists the following therapies as preferred treatments:
Abbreviations:
- CTP – Child-Turcotte-Pugh (score used to assess severity of cirrhosis)
- IU/mL – International Units Per Milliliter
- PEG-IFN/IFN – Peginterferon/Interferon
- RAS – Resistance-associated substitutions
Genotype 1:
Treatment-naïve without or with cirrhosis (CTP A):
Pangenotypic regimens
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens:
Zepatier: 1 tablet orally daily for 12 weeks if GT1a without baseline NS5A RAS or GT1b
Harvoni: 1 tablet orally daily
If HCV-noninfected, non-cirrhotic, and HCV RNA baseline <6 million IU/mL: 8 weeks
If cirrhotic, baseline HCV RNA ≥6 million IU/mL, HIV/HCV-co-infected, or African American: 12 weeks
Consider adding ribavirin in CTP A patients
Treatment-naïve with decompensated cirrhosis (CTP B or C):
Harvoni: 1 tablet orally daily + ribavirin (600 mg/day and increase by 200 mg/day every 2 weeks only as tolerated) for 12 weeks
Epclusa: 1 tablet orally daily + ribavirin (1000 mg/day - <75kg – or 1,200 mg daily - ≥75kg – orally daily in 2 divided doses with food) for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb).
Treatment-experienced (NS5A- and SOF-naïve [e.g., failed PEG-IFN/RBV ± NS3/4A PI]) without or with cirrhosis (CTP A)
Pangenotypic regimens:
Mavyret: 3 tablets orally daily with food
If PEG-IFN/RBV-experienced: 8 weeks if non-cirrhotic or 12 weeks if cirrhotic
If NS3/4A PI + PEG-IFN/RBV-experienced: 12 weeks
Vosevi: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens
Zepatier: 1 tablet orally daily for 12 weeks if GT1b, or if failed only PEG-IFN/RBV and GT1a without baseline NS5A RAS
Harvoni: 1 tablet orally daily for 12 weeks
Treatment-experienced (NS5A-naïve and SOF-experienced) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food
If PEG-IFN/RBV + Sovaldi-experienced: 8 weeks if non-cirrhotic or 12 weeks if cirrhotic
If Olysio + Sovaldi-experienced: 12 weeks
Epclusa: 1 tablet orally daily for 12 weeks if GT1b
Vosevi: 1 tablet orally daily with food for 12 weeks if GT1a
Treatment-experienced (prior NS5A-containing regimen) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 16 weeks if failed only an NS5A inhibitor without NS3/4A PI (e.g., Harvoni)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-experienced with decompensated cirrhosis (CTP B or C)
Epclusa: 1 tablet orally daily + RBV; start at lower RBV doses as clinically indicated (e.g., baseline Hgb);
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks; NOT FDA approved for 24 weeks
Genotype 2:
Treatment-naïve or treatment-experienced (PEG-IFN/IFN ± RBV or Sovaldi + RBV ± PEG-IFN) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks; 12 weeks if CTP A and treatment-experienced or in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-naïve or treatment-experienced patients with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks
Genotype 3:
Treatment-naïve without cirrhosis or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks if cirrhotic or in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
If CTP A, test for NS5A RAS
Add ribavirin if Y93H RAS present
Treatment-experienced (PEG-IFN ± RBV or Sovaldi + RBV ± PEG-IFN) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 16 weeks
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
If CTP A, consider adding ribavirin (no supporting data)
Treatment-naïve or treatment-experienced with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks
Genotype 4:
Treatment-naïve without or with cirrhosis (CTP A)
Pangenotypic regimens
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens
Zepatier: 1 tablet orally daily for 12 weeks
Harvoni: 1 tablet orally daily for 12 weeks
Treatment-naïve with decompensated cirrhosis (CTP B or C)
Pangenotypic regimen
Epclusa: 1 tablet orally daily + RBV for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
Non-pangenotypic regimen:
Harvoni: 1 tablet orally daily + ribavirin (600 mg/day and increase by 200 mg/day every 2 weeks only as tolerated) for 12 weeks
Treatment-experienced (Sovaldi-experienced and NS5A-naïve) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks if NS3/4A PI-naïve without cirrhosis, and 12 weeks if NS3/4A PI-experienced or CTP A
Epclusa: 1 tablet orally daily + ribavirin for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-experienced with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks; NOT FDA approved for 24 weeks
5. PATIENT ASSISTANCE PROGRAMS
The drug manufacturers and various national nonprofit organizations offer a variation of patient assistance programs (PAPs) to assist patients in accessing treatments. They include:
Support Path (Gilead Sciences):
Financial Assistance
Provides Co-Pay Coupons for Sovaldi, Harvoni, Harvoni (Generic), Epclusa, Epclusa (Generic), and Vosevi
Co-Pay Coupons cover out-of-pocket costs up to 25% of the catalog price of a 12-week regimen (3 bottles/packages) of Sovaldi, Harvoni, Harvoni (Generic), Epclusa, Epclusa (Generic), or Vosevi
Excludes patients enrolled in Medicare Part D or Medicaid
Insurance Support
Researches and verifies patient’s benefits, and gives information they need about coverage options and policies
Explain Prior Authorization process and works with HCV Specialist’s office so they can submit PA forms to a patient’s insurance company
May be able to provide assistance with appeals process
Website: http://www.mysupportpath.com/
AbbVie Mavyret Co-Pay Savings Card:
Financial Assistance
Patient may be eligible to pay as little as $5
Excludes patients enrolled in Medicare Part D, Medicare Advantage, Medigap, Medicaid, TRICARE, Department of Defense, or Veterans Affairs programs)
NeedyMeds:
NeedyMeds Drug Discount Card
Designed to lower cost of prescription medications by up to 80% at participating pharmacies
Price finder tool for the drug discount card
No eligibility requirements
CANNOT be used in combination with government healthcare programs, but CAN be used IN PLACE of program
CANNOT be combined with other offers
Website: http://ow.ly/fEJo309cJ7Z
The Assistance Fund:
Status: WAITLISTED
Requires provider referral
Copay assistance
Eligibility Criteria:
US citizen or permanent resident
Diagnosed with the disease for which you are applying
Prescribed an FDA-approved treatment for the disease
Have prescription coverage for the prescribed treatment
Meet financial eligibility criteria based upon household income and size
Patient Advocate Foundation Co-Pay Relief:
Status: CLOSED
Maximum award of $15,000
Eligibility Requirements:
Patient must be insured, and insurance must cover prescribed medication
Confirmed HCV diagnosis
Reside and receive treatment in the U.S.
Income falls below 400% of FPL with consideration of the Cost of Living Index (COLI) and the number in the household
Patient Access Network (PAN) Foundation:
Status: OPEN
Co-Pay Assistance with a maximum award of $6,000
Patients may apply for a second grant during their eligibility period subject to availability of funding
Eligibility Requirements:
Must be being treated for HCV
Have insurance that covers HCV prescribed medication
Medication must be listed on PAN’s list of covered medications: https://www.panfoundation.org/index.php/en/patients/medications-covered
Income falls below 500% of FPL
Residing and receiving treatment in the U.S. (citizenship NOT required)
Website: https://www.panfoundation.org/index.php/en/patients/assistance-programs/hepatitis-c
HealthWell Foundation:
Status: OPEN
Co-Pay Assistance with a maximum award of $30,000
Minimum Co-Pay Reimbursement Amount: None
Minimum Premium Reimbursement Amount: None
Eligibility Requirements:
Must be being treated for HCV
Have insurance that covers HCV prescribed medication
Income falls below 500% of FPL
Receiving treatment in the U.S.
Website: https://www.healthwellfoundation.org/fund/hepatitis-c/
6. HARM REDUCTION PROGRAMS
Harm Reduction, as it relates to opioid abuse and HCV, are measures designed to serve as preventive or monitoring efforts in combating opioid prescription drug and heroin abuse, and as an effect, helping to prevent the spread of HCV and HIV. The Co-Infection Watch covers the following measures: Syringe Exchange, Expanded Naloxone Access, Good Samaritan Laws, Mandatory PDMP Reporting, Doctor Shopping Laws, Physical Exam Requirements, ID Requirements for Purchase, Required or Recommended Prescriber Education, and Lock-In Programs (Editor’s Note: Program descriptions provided herein).
January 2023 Updates:
Syringe Exchange
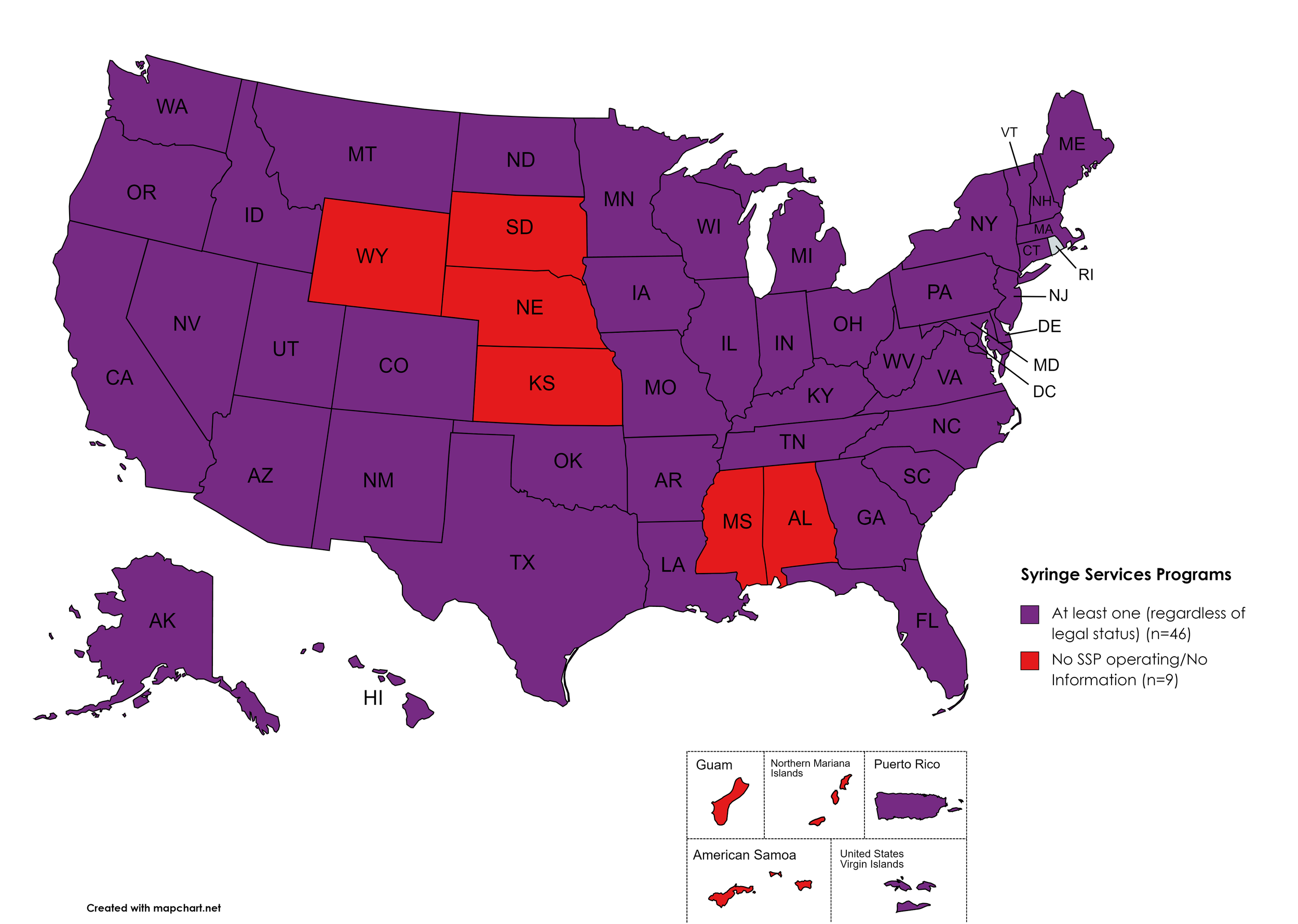
Syringe Services Programs (SSPs) exist to provide injection drug users (or those whose prescriptions require injection) with clean syringes and/or in exchange for used ones. (N.b. – states listed as "at least one SSP…” indicate only that a Syringe Services Program (SSP) exists within the state, regardless of the legality of SSPs under state law).
States with Syringe Exchange: AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MO, MT, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, D.C.
States without Syringe Exchange: AL, KS, MS, NE, SD, WY
Territories with Syringe Exchange: Puerto Rico, U.S. Virgin Islands
Figure 21. January 2023 Syringe Exchange Coverage
Map Key: Purple = Syringe Exchange(s); Red = No Syringe Exchange(s); Grey = No Information
Expanded Naloxone
Naloxone is a drug used to counteract the effects of opioid overdoses. Expanded Access refers to one of more of the following conditions: Naloxone purchase without a prescription; availability to schools, hospitals, and emergency response units for use in the event of an overdose.
States with Expanded Naloxone: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Expanded Naloxone: None
Territories with Expanded Naloxone: Unknown
Figure 22. January 2023 Expanded Naloxone Coverage
Map Key: Purple = Expanded Naloxone; Red = Restricted Naloxone; Gray = No Information
Good Samaritan Laws
Good Samaritan Laws are laws that are designed to protect emergency services personnel, public or private employees, and/or citizens from being held legally liable for any negative healthcare outcomes as a result of providing "reasonable measures" of emergent care.
States with Samaritan Laws: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Samaritan Laws: None
Territories with Samaritan Laws: Unknown
Figure 23. January 2023 Good Samaritan Laws Coverage
Map Key: Purple = Good Samaritan Laws; Red = No Good Samaritan Laws; Gray: No Information
Mandatory PDMP Reporting
Prescription Drug Monitoring Programs (PDMPs) are programs established by state and/or federal law that requires prescribing physicians and the fulfilling pharmacies to report to a state agency one or more of the following data points: Patient Names; Specific Drug(s) Prescribed; Prescription Dosage; Date; Time; Form of State-Issued ID.
States with PDMP Reporting: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without PDMP Reporting: MT, SD
Territories with PDMP Reporting: Guam
Figure 24. January 2023 Mandatory Prescription Drug Monitoring Program Coverage
Map Key: Purple = Mandatory PDMP; Red = No Mandatory PDMP; Gray = No Information
Doctor Shopping Laws
Doctor Shopping Laws are those laws designed to prevent patients from seeking one or more of the same prescription from multiple doctors through the use of subterfuge, falsifying identity, or any other deceptive means. While federal law prohibits Doctor Shopping, most states also include provisions that prohibit patients from seeking a new prescription if another physician has denied a similar prescription within a certain period of time.
States with Doctor Shopping Laws: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Doctor Shopping Laws: None
Territories with Doctor Shopping Laws: None
Figure 25. January 2023 Doctor Shopping Laws Coverage
Map Key: Purple = Doctor Shopping Laws; Red = No Doctor Shopping Laws; Grey = No Information
Physical Exam Required
Physical Exam Requirements are those that mandate that the prescribing physician perform a physical examination on a patient before providing a prescription for a controlled substance to determine if the prescription is medically necessary.
States with Physical Exam Required: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KY, LA, MD, MA, ME, MI, MN, MO, MS, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, PA, RI, SC, TN, TX, UT, VA, VT, WA, WV, WY, D.C.
States without Physical Exam Required: KS, MT, OR, SD, WI
Territories with Physical Exam Required: None
Figure 26. January 2023 Physical Exam Required Coverage
Map Key: Purple = Physical Exam Required; Red: No Physical Exam Required; Grey = No Information
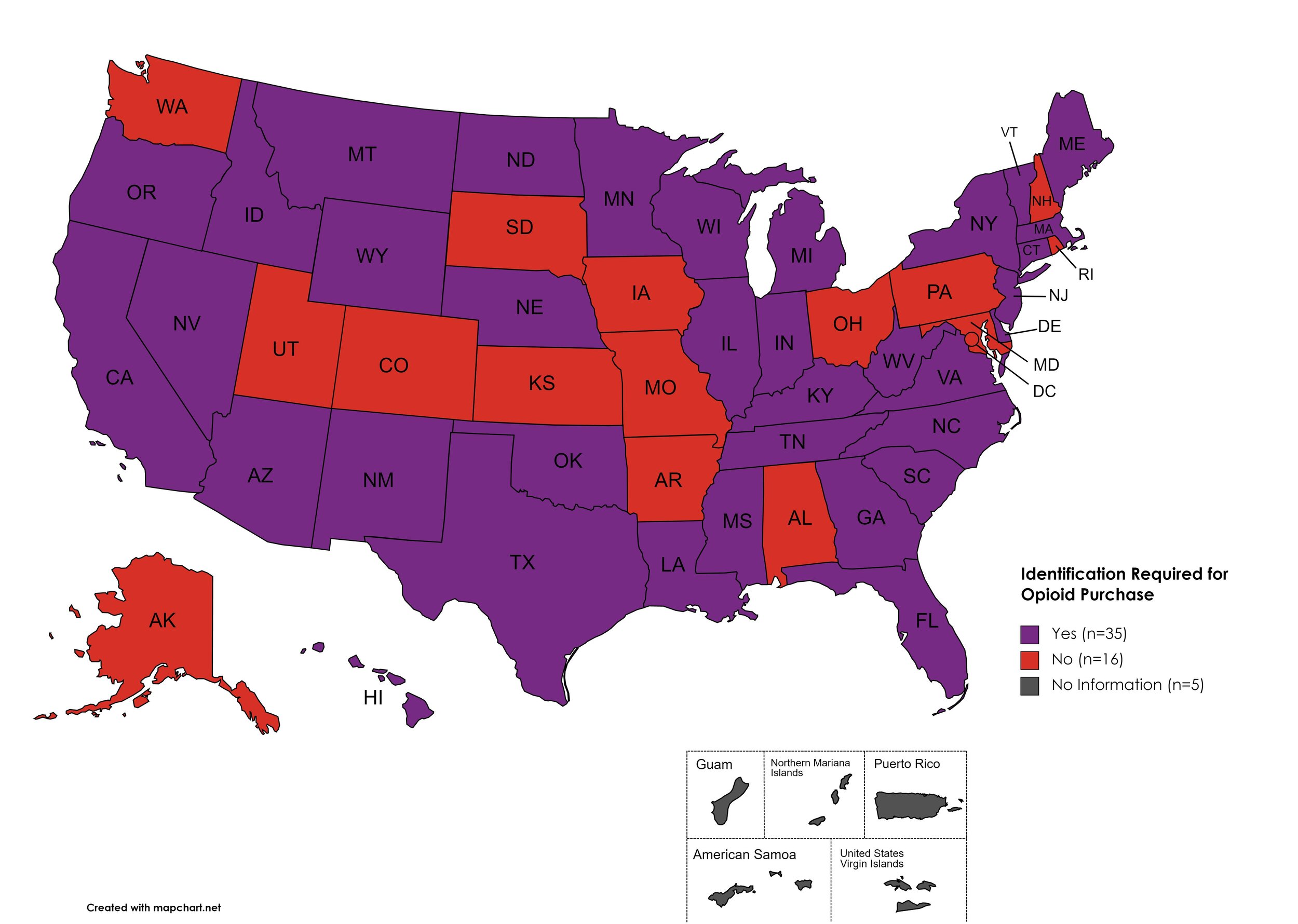
I.D. Required for Purchase of Opioid Prescription
Federal law requires anyone purchase a controlled substance to provide a state-issued identification (“I.D.”) in order to fill the prescription. Mandatory ID requirements go further and require that this information be recorded and stored in an effort to prevent the same patient from obtaining multiple or repeated prescriptions in a given period of time.
States with I.D. Required: AZ, CA, CT, DE, FL, GA, HI, ID, IL, IN, KY, LA, ME, MA, MI, MS, MN, MT, NE, NV, NJ, NM, NY, NC, ND, OK, OR, SC, TN, TX, VT, VA, WV, WI, WY
States without I.D. Required: AL, AK, AR, CO, IA, KS, MD, MO, NH, OH, PA, RI, SD, UT, WA, D.C.
Territories with I.D. Required: Unknown
Figure 27. January 2023 I.D. Required Coverage
Map Key: Purple = I.D. Required; Red = No I.D. Required; Gray = No Information
Prescriber Education Required/Recommended
States that require/do not require that prescribing physicians undergo special training related to safer prescribing and utilization practices.
States with Prescriber Education Required: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MS, NE, NV, NH, NJ, NM, NY, NC, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Prescriber Education Required: KS, MO, MT, ND, SD
Territories with Prescriber Education Required: Unknown
Figure 28. January 2023 Prescriber Education Required Coverage
Map Key: Purple = Prescriber Ed Required; Red = No Prescriber Ed Required; Gray = No Information
Medicaid Lock-In Program
Lock-In Programs are laws requiring that patients either receive prescriptions from only one physician and/or fill prescriptions from only one pharmacy.
States with Medicaid Lock-In Program: AL, AK, AZ, AR, CA, CO, CT, DE, GA, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Medicaid Lock-In Program: FL, HI, SD
Territories with Medicaid Lock-In Program: Unknown
Figure 29. January 2023 Medicaid Lock-In Coverage
Map Key: Purple = Medicaid Lock-In; Red = No Medicaid Lock-In; Gray = No Information
January 2023 Notes:
MO has begun implementation of PDMP reporting. Legislation was passed in 2021, however the statewide program did not begin implementation immediately.
CANN is no longer able to independently verify the existence of an SSP in Kansas. KS state laws prohibit SSPs and syringes are included in the state’s drug paraphernalia law.
7. COVID-19 IMPACT ON HIV & HCV
The Community Access National Network’s blog began 2021 by assessing COVID-19’s impact on HIV, HCV, and Substance-Use Disorder. We've subsequently followed-up by asking, COVID-19: How Far We’ve Come & How Far We Have to Go? We continue to monitor developments in light of the ongoing COVID-19 pandemic and its impacts on public health.
Additional Resources and Relevant Issues:
Declaration of Public Health Emergency Renewed - On January 11, 2023, the U.S. Department of Health and Human Services (HHS) Secretary Xavier Becerra renewed the existing declaration of a public health emergency (PHE) due to COVID-19. The previous declaration was set to expire in April 2023. To review some potential changes when the PHE ends, click here.
CDC: HIV Services and Outcomes During the COVID-19 Pandemic - United States, 2019-2021 - In a Morbidity and Mortality Weekly Report (MMWR) issued December 2, 2022, the U.S. Centers for Disease Control and Prevention (CDC) evaluated the impact of the COVID-19 pandemic on the quantity and some quality aspects of HIV services in public health. Overall, the report found that HIV testing and number of people prescribed pre-exposure prophylaxis (PrEP) decreased in the first two quarters of 2020 and, mostly, rebounded by the third quarter. Generally speaking, those persons living with HIV considered linked to care, prescribed antiretroviral therapy, and having achieved viral suppression stayed stable during the study period.
COVID-19 and People Living with HIV - On January 10, 2023, HIV.gov updated their resource page regarding COVID-19 and people living with HIV. The update includes more resource materials on both HIV and COVID-19, referencing federal programs and actions, and additional data on vaccine safety and efficacy for PLWHA.
HIVMA Creates Paxlovid Clinical Resources for Treating People Living with HIV and Hepatitis C - The HIV Medicine Association published guidance for treating PLWHA and Hepatitis C for COVID-19 with Paxlovid toward the end of 2022, as the United States faced the threat of another wave of COVID-19 cases. While much of the guidance reiterates the information on the U.S. Food and Drug Administration’s fact sheet, the brief is more direct. Because one of the ingredients to Paxlovid is the ritonavir, those patients on a protease inhibitor regimen for the treatment of HIV should be monitored for an increase in potential adverse events. For people living with Hepatitis C, similarly, they should be monitored. However, there’s an exception; Paxlovid is not recommended for patients on glecaprevier/pibrentasvir, sold under the brand name Mavyret, which happens to be one of the most accepted direct acting agents for all HCV genotypes.
Fewer Medi-Cal Patients Got Hepatitis C Treatment Amid COVID - In fiscal years 2018-19 and 2020-21, the number of Medi-Cal patients who received Hepatitis C treatment dropped by about 40%, despite the state having removed barriers like prior authorizations. Experts cited in the Times Post article shared a concern that “easy to reach” populations who may be at risk for acquiring HCV have “already been tapped” and programmatically, the state does not address follow up and linkage to care for HCV the way it does other infectious diseases. The sentiment that things only “get harder” from here is well founded, given stagnating rates of curative treatment in states like Louisiana, where cost control programs have essentially removed the barrier to financial access but other structural supports remain lacking.
High Flu Activity, COVID-19, and Hepatitis A Cases Continue: Virginia Health Officials Say - In late December 2022, officials from the Roanoke and Alleghany Health Districts in Virginia shared the Hepatitis A outbreak that started in January 2022 was still ongoing, having already hospitalized 63 Virginians. New COVID-19 and influenza cases were also high, relative to previous months, and urged Virginians to get vaccinated. Of particular note, of the 37 people hospitalized for COVID-19 at the time of reporting, 23 of those reported as new hospitalizations on the same day. Despite national monitoring trends downward on new COVID-19 cases and hospitalizations, COVID-19 deaths rose significantly in the first two weeks of January 2023.
A Study Aims to Determine Whether Longer-Term Paxlovid Can Mitigate Long COVID - The Body’s Tim Murphy covers the experience of a person living with HIV, chronic fatigue syndrome and…long COVID, in the context of a new study which aims to divine if a longer course of the antiviral Paxlovid can alleviate the symptoms some COVID patients find experiencing for days, weeks, and even months after their initial bout with the virus. Highlighting that nearly 20% of all COVID patients in the United States report experiencing some form of symptomology post-acute SARS-CoV-2 infection, if the National Institutes of Health (NIH) study does end up with positive findings for a longer course of the medication, there might be some hope for those having to manage the host of symptoms that make up the diagnosis. Most commonly, long COVID is characterized by persistent fatigue," “brain fog”, and shortness of breath but the symptoms can be far more diverse than these, including higher incidence rates of hypertension, heart attack, stroke, and embolsim. Because of the history some patients have with providers dismissing chronic fatigue as “all in your head”, advocates warn resources allocated by congress should focus on investigating potential treatments, not exclusively on mental health support because, while that mental health support helps patients navigate their symptoms, it doesn’t actually address the symptoms and could easily fall into the trap of considering a the very real physical illness of long COVID as a mental illness which does not need specific treatments.
8. LATEST NEWS
****IMPORTANT**** TENNESSEE TO CUT FEDERAL HIV FUNDING - CANN is closely monitoring the situation developing in Tennessee after the state announced it would be rejecting future federal funding from the U.S. Centers for Disease Control and Prevention (CDC) for programs designed to prevent and monitor new HIV diagnoses and dollars tied to the federal initiative known as Ending the HIV Epidemic (EHE). National advocates are particularly concerned about the abrupt nature of the announcement and how this situation might be a warning, should other states follow suit. The move comes after Tennessee engaged in efforts to oust prevention services contractor, Planned Parenthood, and a federal judge enjoined the state from doing so in 2012. The injunction still exists today and it is not entirely clear the move from the state will inherently change the first amendment issues at the center of the injunction. State officials said the rejection of federal dollars was centered around the idea of “reducing federal dependency” and future prevention programs would be funded by state dollars with state priorities focusing on first responders, preventing vertical transmissions, and human trafficking victims. For clarity, the CDC reports zero occupational transmissions among health care providers since 2013 and Tennessee’s own 2020 epidemiological report found zero new perinatal transmissions of HIV in 2019.
Renewal of Determination That a Public Health Emergency Exists - On December 22, 2022, the U.S. Department of Health and Human Services (HHS) Secretary Xavier Becerra renewed the ongoing declaration of public health emergency regarding the opioid crisis. The declaration is the latest in extensions regarding the opioid crisis, originating in October 2017.
Monkeypox Response Project for People Living with HIV - New Update Available - On December 8th, new updates were posted as part of the ongoing Monkeypox (MPV) Response Project for People Living with HIV. The updates include: 1) report on A Patient's Guide to MPV: Report No. 3, 2) infographic on Monkeypox (MPV) & HIV, and 3) blog on Troubling Issues with HIV and Monkeypox Co-Infection. An updated report — accompanied by a new infographic and blog — will be made available later this week, and it will be posted here. The project is generously supported by Gilead Sciences.
Statement from HHS Secretary Becerra on MPOX On December 2, 2022, Health and Human Services Secretary, Xavier Becerra, issued a statement indicating the agency will not likely renew the Public Health Emergency declaration for Monkeypox (MPV, MPOX) when it is set to expire on January 31, 2023.
Janssen and Partners Discontinue Mosaico HIV Vaccine Trial - One of the most promising, late stage HIV vaccine trials to date has come to a close after trial findings concluded efficacy was lacking even while the same work found the product was safe. The Mosaico trial enrolled about 3,900 participants from vulnerable populations at 51 different rial sites. The vector for the vaccine sought to build off of Janssen’s COVID-19 vaccine design, using an adenovirus serotype backbone and would have been administered as a four dose series across a year. Dr. Stephaun Wallace of the HIV Vaccine Trials Network said while the outcome of the trial was disappointing, it wasn’t a complete loss. Data gleaned from the trial will inform future work. One of the challenges HIV vaccine candidate trials face is participant recruitment, as the relatively wide availability of pre-exposure prophylaxis (PrEP), especially longer acting products, makes teasing out efficacy of a vaccine from efficacy of PrEP (and ethical issues in trial design quire efforts to ensure participants are not unnecessarily put at risk for contracting HIV). Dr. Wallace concluded that a robust options future would be critical, where both PrEP and a vaccine were made available to meet the needs of highly affected communities.
White House Announces Federal Evidence Agenda on LGBTQ+ Equity - On January 24, 2023, the Biden Administration released the the first-ever federal evidence agenda on LGBTQ+ equity, focused on developing more robust and inclusive data gathering and handling policies to accurately measure the state of LGBTQ+ quality of life and discrimination in the United states. The intra-agency agenda seeks to modernize and include LGBTQ+ persons in data collection activities around health, housing, employment, school access, and more. Data gathering for this highly affected population has been rife with issues in years past, largely dependent on data gathered by academic institutions and community-based organizations. The data gathering effort will help the federal government in developing policies and programs in order to address the needs of LGBTQ+ people across the nation, similar to data gathering on other marginalized populations. Key in the agenda is a desire to maintain identity security of participants of which personal data is gathered.
Antibiotic-Resistant Gonorrhea Strain Detected in Mass. Raising Concerns - The Massachusetts Department of Public Health (MDPH) has announced the detection of a novel strain of gonorrhea with extraordinary resistance to multiple drug therapies. While drug-resistant gonorrhea has been a concern for many years now, this is the first case of resistance or reduced response to five classes of antibiotics in the United States. MDPH issued a health alert to clinicians, remind them of the necessity to submit laboratory samples for resistance testing to the state’s public health surveillance library and about appropriate treatment of presumptive and positively confirmed cases of gonorrhea. Antibiotic stewardship has been a topic of deep discussion in the United States as more antimicrobial resistance continues to present challenges in an area of treatment which is marred by underinvestment.
Study: Impact of Hepatitis C Virus Point of Care RNA Viral Load Testing Compared with Laboratory-Based Testing on Uptake of RNA Testing and Treatment, and Turnaround Times - As the United state evaluates effective development and implementation of Hepatitis C point-of-care screening and testing as a method of removing barriers to care for patients, the World Health Organization led a study published on January 24, 2023 in The Lancet. The study, a meta-analysis of previous works, sought to evaluate the differences between already available point of care testing for HCV elsewhere in the world in terms of turnaround times and patient-centered metrics, like successful initiation of curative treatment. The study found a positive correlation between point of care testing, as opposed to laboratory-based testing, in turnaround times between testing and treatment uptake and increased overall treatment uptake. The analysis also sought to distinguish any differences between same-site models of testing and treatment and different-site models. In same-site models, patients were more likely to engage in treatment. The implications of the study specifically point toward a need for mobile and off-site clinics engaging in point of care testing to also provide immediate access to treatment for patients, when appropriate. A streamlined model of care poses as a potential game-changer in the effort to eliminate HCV globally.
Medicare Invites Public Comment as it Considers National Coverage Determination for HIV PrEP - The Centers for Medicare and Medicaid Services (CMS) has opened a call for public comment on the potential of issuing a national coverage determination for PrEP. Comments can be issued here. The move from CMS seeks to integrate PrEP coverage as part of covering “additional preventative services” under the Social Security Act. This public comment period, which ends on February 11, 2023, comes at the urging of ViiV Healthcare* after the development and approval of Apretude, the long-acting injectable medication for PrEP, and the United States Preventative Services Taskforce (USPSTF) began amending its 2019 grade A rating for PrEP to include all FDA approved PrEP medications, rather than just Truvada. The USPSTF update, which began in December 2022, is yet still pending but not expecting particular pushback and advocates have praised the move to encompass additional medications and innovation.
*ViiV Healthcare is a funding partner to CANN.