Beyond the Test: Ensuring Linkage to Care After HIV Self-Testing
HIV self-test kits have emerged as a pivotal tool in the fight against HIV/AIDS, offering a private and convenient method to determine one's status. The World Health Organization endorsed these kits in 2016, marking a significant step in global HIV/AIDS prevention. The COVID-19 pandemic further highlighted their importance as traditional testing declined, with the Centers for Disease Control & Prevention (CDC) emphasizing their role in ensuring continued access to HIV testing.
In the U.S., 14% of the estimated 1.1 million people living with HIV are unaware of their status. Men who have sex with men (MSM) have a particularly high prevalence of undiagnosed men with sero-positive status. Yet, a study in JAMA Network found that only 25.7% of MSM in urban areas had used an HIV self-test. This limited adoption and data indicating that many don't pursue necessary treatment or prevention post-testing, highlights the challenges ahead.
HIV Self-Testing: Nuances and Linkage to Care
HIV self-test kits, endorsed for their easy access to HIV testing, come with detailed instructions for self-administration and result interpretation. However, users are strongly advised to verify their results at a healthcare facility, particularly if they end up with a reactive (“positive”) result.
While the potential of HIV self-testing is evident in its ability to increase the number of people aware of their HIV status, the real challenge lies in ensuring that those who test positive are seamlessly linked to appropriate medical care and support. A study in The Lancet highlighted a significant gap in connecting these individuals to post-testing HIV care. This gap is alarming, considering the importance of early intervention in HIV management, which not only benefits a person’s health but also reduces the risk of transmission.
A systematic review by The Conversation further emphasized this challenge:
There's an 8% increase in users finding an HIV clinic post-testing.
A significant number of users did not initiate HIV treatment or PrEP after self-testing.
Women sex workers were 47% more likely to seek medical care post-testing, yet testing rates among clients of sex workers remained unchanged.
MSM users might engage more in condomless anal sex post self-testing.
One major obstacle in this linkage to care is the lack of localized resources accompanying the test kits. For example, kits from OraSure, a leading manufacturer, provide general post-test advice but often lack specific resources or directions for localized care, leaving people, especially those testing positive, uncertain about their next steps.
To address these challenges, it's crucial to not only link people to care but also ensure they access the necessary treatment and preventive measures. Strategies that have proven effective in bridging care gaps for chronic conditions, like hepatitis B, can be adopted. Leveraging community-based participatory approaches, partnering with community organizations, and implementing robust referral systems can ensure that people receive the essential care and support post-testing.
Benefits, Hurdles, and Real-World Implications of Self-Testing
HIV self-testing offers a private and convenient alternative to traditional methods, addressing barriers such as transportation, stigma, confidentiality concerns, and outdated HIV criminalization laws. A Vital Signs report from 2016 revealed that 38% of new HIV transmissions were from people who were unaware of their status, emphasizing the need for increased testing. The CDC's eSTAMP study found that self-tests doubled the likelihood of MSM identifying new HIV transmissions.
However, challenges persist. Many users, despite recognizing their status, don't or can’t take subsequent necessary steps, such as pursuing HIV treatment or initiating PrEP, as highlighted in The Conversation. Additionally, functional considerations like storage conditions play a role in the effectiveness of self-tests. For instance, the OraQuick test should be stored between 36°F and 80°F, a factor that becomes increasingly relevant in the face of climate change, hot summers, and extended transit times. Similarly, self-testing kits produce physical evidence of screening that needs to be discarded by the person using the test. If that person is in a safe, welcoming situation, storing the test or disposing of the materials from the test might result in risks of experiencing stigma, discrimination, or even harm.
Accuracy in self-testing is paramount. The OraQuick In-Home HIV Test claims over 99% accuracy for negative results and 91.7% for positive ones, though the testing window period can influence accuracy. Users appreciate the autonomy self-testing offers, but it should be part of a broader strategy, complemented by counseling and care linkage and stigma reduction, as emphasized by The Lancet study.
Financial Incentives and Real-Life Implications:
In many studies evaluating the effectiveness and adoption of HIV self-tests, participants were often provided financial incentives to report their test results. This approach ensured a higher rate of result reporting and offered insights into user behavior post-testing. However, in real-life scenarios, such financial incentives are absent. This discrepancy raises concerns about the actual rate of result reporting and subsequent linkage to care when people purchase and use these kits outside of a study environment. Without the motivation of financial incentives, there's a potential risk that some people might not seek further care or counseling after a reactive test, especially if they lack access to localized resources or support systems.
Economic Factors, Barriers, and the Way Forward
Self-testing presents a promising avenue to increase HIV status awareness, but economic and psychological barriers hinder its adoption. The CDC found that 61% of Americans had never been tested for HIV, and less than 30% of those most at risk had been tested in the preceding year.
The CDC's eSTAMP study highlighted the effectiveness of mailing free self-tests, with recipients being more likely to discover their HIV status. Such initiatives are cost-effective, with potential savings of nearly $1.6 million in lifetime HIV treatment costs, as estimated from the eSTAMP trial.
Despite these advantages, challenges like misconceptions about HIV risk, unawareness of self-tests, and cost considerations persist. Additionally, the emotional toll of a reactive result, especially when received alone, is a significant concern. The CDC's efforts to make HIV self-testing more accessible are commendable and addressing these barriers is essential for the initiative's success.
Conclusion
HIV self-testing is a crucial and beneficial tool in our ongoing fight against the HIV/AIDS epidemic. Yet, as with any innovative solution, it's not without its challenges. The true measure of our commitment to Ending the HIV Epidemic lies not just in the tools we develop but in the systems we put in place to ensure their effective use. As we embrace the promise of self-testing, we must also confront the gaps in linkage to care, address the barriers to widespread adoption, and ensure that every person and community, regardless of background, has the support they need post-testing.
We must ask ourselves: Are we doing enough? Are we truly leveraging the potential of these tools to make a lasting impact? The answers to these questions will shape the trajectory of our fight against HIV/AIDS.
Healthcare advancements are made every day, and it's our collective responsibility to ensure these innovations reach and benefit all, especially the most vulnerable among us. As we move forward, let's commit to not only advocating for the tools but also for the comprehensive systems of support that make them truly transformative. Because in the end, it's not just about testing; it's about reducing stigma, saving lives, building healthier communities, and creating a future free from the shadow of HIV/AIDS and that’s worth investing in.
Payer-Denied PrEP Fails Black Women and Marginalized Communities
In the battle against HIV, Pre-exposure Prophylaxis (PrEP) stands out as a transformative defense, significantly lowering infection risks for those most vulnerable. However, this critical protection remains alarmingly out of reach for many, especially Black women, due to insurance payers' denial of coverage. This systemic neglect transcends a mere healthcare gap; it's a stark reflection of the health disparities that exist in the United States’ healthcare construct.
Recent findings from IDWeek 2023, led by Li Tao, MD, PhD, confirm: obstacles to PrEP, particularly insurance denials, are directly linked to rising HIV diagnoses. The research, spanning January 2019 to February 2023, exposes a distressing reality where people with rejected PrEP claims encountered a 95% higher likelihood of new HIV diagnoses compared to recipients of the medication.
Moreover, delays in PrEP dispensing due to these denials correlated with approximately a 20% increased HIV contraction risk, emphasizing the urgency of immediate PrEP access. This isn't just a postponement; it's a life-threatening denial disproportionately affecting marginalized communities. The study highlights the necessity of prompt PrEP access to prevent new HIV infections, especially for those with rejected or abandoned claims.
Further analysis showed the lowest HIV diagnoses among cisgender men with dispensed claims, contrasting with the highest rates among transgender women and men with abandoned claims. Individuals with sexually transmitted infections in the rejected or abandoned groups also faced elevated HIV diagnosis rates.
These insights "emphasize the dire need to eliminate PrEP access barriers to halt HIV transmission," the researchers concluded.
Empowering People: The Psychological Benefits of PrEP
PrEP's impact extends beyond physical health, offering significant psychological relief. A recent study in Pharmacy Times illustrated that PrEP usage enhances the confidence of people in having safer sex, reducing HIV transmission anxiety. This security is crucial, especially for communities burdened by the constant dread of HIV. It represents not just a medical breakthrough but an empowerment tool, allowing people to regain control over their sexual health without looming HIV fears.
The study, conducted over 96 weeks, encompassed participants from various backgrounds, including men who have sex with men, transgender women, and cisgender women. It found that those on PrEP experienced less anxiety and more comfort during sexual activities, confident in their reduced HIV risk. This mental health benefit was consistent across all groups, highlighting PrEP's universal advantage beyond its physical protective effect.
"PrEP is more than a medical solution; it's a source of hope and assurance for those at elevated risk of HIV," the researchers noted. They suggested that this confidence might encourage adherence to the medication, strengthening prevention efforts.
However, when such empowering medical solutions are restricted, it doesn't just withhold a health service; it robs people of the mental peace that accompanies protection. This added psychological strain compounds the systemic injustices that marginalized communities endure, deepening disparities.
Global Perspectives on HIV Prevention
While the U.S. struggles with healthcare inequities, other countries are advancing in the HIV fight. For example, Australia has made significant strides by implementing a comprehensive HIV prevention approach. A pioneering study in The Lancet HIV demonstrated that integrating HIV treatment-as-prevention (TasP) with PrEP significantly reduced new HIV cases among gay, bisexual, and other men who have sex with men (GBM).
This extensive research in New South Wales and Victoria, Australia's most populous states, indicated a substantial rise in the population prevalence of viral suppression, accompanied by a corresponding decline in HIV incidence. The findings advocate for continuous investment in holistic HIV prevention strategies, suggesting that even slight enhancements in treatment and prevention access and adherence can drastically reduce new HIV cases.
However, Australia's success highlights a stark contrast in healthcare access and strategy in the U.S., especially impacting Black women and marginalized communities. The effectiveness of Australia's model stems from its inclusivity, guaranteeing extensive coverage and straightforward access to diagnostic and treatment services. This strategy encompasses not just broad PrEP availability but also a robust focus on TasP, ensuring a high treatment rate among those diagnosed with HIV, thereby lowering their viral load and transmission risk.
In contrast, the U.S. healthcare system's piecemeal strategy, characterized by payer denial for PrEP and other preventive measures, hampers these all-encompassing prevention methods. A Health Affairs study unveiled severe disparities in PrEP access and costs. In 2018, populations such as gay, bisexual, and same gender loving men (GBM), heterosexual women and men, and people who inject drugs encountered the most significant financial barriers. Insurance plays a crucial role in healthcare access, yet it's grossly inadequate regarding PrEP coverage. According to a 2022 study, numerous people encountered administrative barriers, including outright PrEP claim denials. These systemic shortcomings resulted in uncovered costs totaling an astonishing $102.4 million annually, a financial burden that individuals had to shoulder in 2018 alone.
These uncovered costs represent tangible hurdles, keeping potentially life-saving medication inaccessible for many at-risk individuals. The systemic obstacles that Black women face, as outlined in a KFF Health News report, emphasize the grave repercussions of this neglect. Economic challenges, healthcare exclusion, and biased marketing strategies limit PrEP access, leaving this community exposed and overlooked.
The current state demands an immediate reassessment of the U.S. HIV prevention strategy. It's not solely about PrEP accessibility; it's about a comprehensive approach that encompasses effective treatment for people living with HIV/AIDS (PLWHA). This strategy involves not just advocating for PrEP insurance coverage but also pushing for extensive healthcare reforms that guarantee all-encompassing coverage, including TasP methods.
Australia's success story provides clear evidence: with the proper dedication and strategic approach, we can substantially lower new HIV transmissions and work towards eradicating the HIV epidemic. However, this achievement requires a collective resolve to seek a healthcare system that serves everyone, not just a privileged minority.
It's time to hold payers and policymakers responsible, to shame them for their role in a system that continues racial and socioeconomic health disparities. Their inaction costs more than money; it costs lives. The U.S. must learn from global success stories and adopt an inclusive, comprehensive HIV prevention strategy that recognizes and caters to the unique needs of all communities, including Black women and other marginalized groups.
The Plight of Black Women
The situation becomes even more tragic when we consider Black women's struggles, especially those identifying as cisgender. Despite bearing a disproportionate burden of the HIV epidemic, Black women face numerous systemic barriers, from healthcare alienation and biased marketing to economic hardships, all limiting their PrEP access, as detailed in a KFF Health News report.
One personal story, that of Alexis Perkins as featured in a PBS NewsHour article, illustrates these challenges. Perkins, a 25-year-old nurse, encountered difficulties in obtaining PrEP despite her proactive health efforts. During her visit to her OB-GYN’s office, Perkins sought a prescription for PrEP but encountered several obstacles. The medical assistant who initially greeted her was not only unfamiliar with PrEP but was uncomfortable discussing it. Furthermore, her provider, though aware of PrEP, did not feel confident prescribing it due to a lack of sufficient knowledge about the medication. Her experience reflects a broader systemic problem where healthcare providers often lack PrEP knowledge or are reluctant to prescribe it, failing to meet Black women's health requirements. "It's not something that's being marketed to us," Perkins expressed in the article, indicating the absence of information directed at Black women.
These systemic barriers extend beyond mere neglect; they inflict direct harm. The research points out exclusionary marketing tactics, where PrEP promotional efforts frequently miss Black women, resulting in misunderstandings and unawareness about PrEP's relevance to their lives. This issue is aggravated by the gender disparity in FDA-approved PrEP medications' accessibility, with some treatments tested solely on male participants, restricting their use for women.
While manufacturers have begun addressing these disparities in marketing materials and clinical trial design for emerging therapies, stigma, radical judicial activism, and lack of strengthened or continued investment in public health all threaten our ability to meet our goals in Ending the HIV Epidemic.
Economic barriers, more common among Black communities, exacerbate these challenges, making consistent PrEP usage difficult. The necessity for regular medical appointments and HIV testing, along with high initiation costs and logistical hurdles, presents significant obstacles. As the PBS article elaborates, these economic and logistical barriers, particularly for those in poverty, are daunting and often insurmountable, barring Black women from the healthcare they deserve.
Addressing this healthcare inequality demands a comprehensive strategy. Economic and social empowerment, community-focused health campaigns, and policy and research initiatives are essential. Improving access to quality employment, healthcare, and stable housing can empower Black women to make informed health decisions. Customized health messaging and community dialogues, as well as policy and research efforts like those by the Centers for Disease Control and Prevention (CDC) and Gilead Sciences, are vital steps toward closing these gaps.
Alexis Perkins' story is not unique; it reflects the experiences of many individuals navigating a healthcare system that consistently fails them. As we face these harsh truths, we must acknowledge that this crisis goes beyond medicine; it's a moral issue. The barriers preventing access to PrEP for the most at-risk communities are not mere oversights; they are expressions of a societal hierarchy that deems certain lives less worthy.
This is more than a health disparity; it's a measure of our societal values. Will we maintain a system that actively undermines the health and futures of its most vulnerable? Or do we possess the collective bravery to demand change?
Change is achievable; it's been proven. Nations like Australia have adopted inclusive, forward-thinking, and compassionate public health policies, dramatically reducing HIV transmission and new diagnoses. Their methods show that with sufficient commitment, we can revolutionize healthcare delivery.
Reflecting on the stories of people like Alexis Perkins, let's contemplate our role in this narrative. Will we be passive observers in a system that discriminates and excludes, or will we become advocates, championing a future where healthcare is a right, not a privilege determined by socioeconomic status? The decision is ours, and it's one we must make now. Because with every moment we delay, every moment we choose inaction, we become silent co-conspirators in a system that tallies casualties instead of champions. In a country that boasts freedom and justice for all, how long will we allow these injustices to determine who gets to live, prosper, and contribute to our society?
Considerations for Hepatitis C Vaccine in HIV-HCV Co-Infected Populations
The interplay between groundbreaking research and its real-world application can shape the trajectory of entire communities. Once of the most evident place we see this is in the realm of HIV-HCV co-infection. As we stand on the precipice of breakthroughs in Hepatitis C Virus (HCV) vaccine development, the unique challenges posed by HIV-HCV coinfection come into sharp focus, reminding us of the urgency and significance of our endeavors.
Understanding the Landscape of HIV-HCV Coinfection:
HIV and HCV coinfection represents both a medical challenge and a reflection of broader societal issues searching for policy solutions. These viruses mainly impact marginalized communities, highlighting deeper socio-economic disparities. The combination of HIV, which taxes the immune system, even when well-controlled, and HCV intensifies health risks, such as liver diseases, emphasizes the need for effective interventions like a preventive HCV vaccine. Beyond the medical perspective, societal barriers like stigma, payer barriers, and limited healthcare access further complicate the issue. Recent vaccination studies, including those for COVID-19 and Hepatitis B Virus (HBV) among people living with HIV (PLWH), underscore these challenges and the necessity for tailored strategies. To comprehensively address HIV-HCV co-infection, a holistic approach that considers both medical and societal aspects is essential.
Drawing Parallels: Vaccination Lessons for HIV Patients:
The vaccination experiences of PLWH, especially during the COVID-19 pandemic, highlight the need for tailored strategies. While HIV patients were prioritized due to potential severe COVID-19 risks, vaccine efficacy varied based on individual immune responses, suggesting the potential need for boosters. Similarly, the Hepatitis B vaccination journey revealed that many PLWH had suboptimal responses to the standard vaccine. However, alternative, additional, or re-administration dosing regimens emerged as a promising solution. As we approach HCV vaccine development for people with co-occurring conditions, these experiences and the data-driven developments originating from them provide invaluable insights to anticipate challenges and innovate solutions.
Special Considerations for Vulnerable Populations:
Equitable policy and programmatic design in public health ensures everyone has access to optimal healthcare, yet societal barriers often sideline certain groups. Incarcerated people face challenges like close-quartered living and limited healthcare access, amplifying the transmission of illnesses like HIV and HCV. Tailored strategies, informed by COVID-19 vaccination efforts in prisons, such as on-site clinics, can improve vaccine uptake. People experiencing homelessness, battling issues like unstable housing and societal stigma, benefit from strategies like mobile clinics and community collaborations, as seen with HBV vaccinations. Building community trust, especially for populations with historical mistrust, is vital. Addressing HIV-HCV coinfection requires an inclusive, trust-centric approach, ensuring no one is overlooked.
Parallels with Mpox Vaccine: Addressing Vulnerable Populations
The U.S. Mpox outbreak in 2022 highlighted health disparities, especially among people experiencing homelessness, LGBTQ+ persons, and people of color. Mpox's transmission and significant impact on gay, bi sexual, and same gender loving men (GBSGLM), including those living with HIV, mirrors challenges with HIV-HCV co-infection.
The outbreak revealed health inequity issues, such as stigma and misinformation, exacerbated by the disease's former name "monkeypox." The Centers for Disease Control and Prevention’s (CDC) Mpox Vaccine Equity Pilot Program and Chicago Health Department's community-centric strategies provided insights for HIV-HCV coinfection management. Key takeaways included:
Community Engagement: Engage with affected communities, fostering trust through tailored programs and partnerships. Ready availability and responsiveness were critical to earned trust among affected communities.
Combatting Stigma: Deliver clear messages to dispel myths, ensuring vaccine uptake.
Vaccine Accessibility: Emphasize genuine accessibility, especially for marginalized groups, inspired by the Mpox Vaccine Equity Pilot Program.
Addressing HIV-HCV Co-infection in Vulnerable Groups:
Equity is vital in managing HIV-HCV co-infection, with incarcerated persons and populations experiencing homelessness and housing instability demanding special focus.
Incarcerated Populations: Prisons, due to their close confines and shared activities, are hotspots for disease transmission. While confinement offers some healthcare delivery opportunities, many lack comprehensive or personalized care, and most-notably, provide a microcosm of healthcare failures affecting surrounding communities. Identifying cost-effective program designs which address these disparate would prove beneficial for communities writ-large. Similarly, ensuring post-release care continuity is essential.
Homeless Populations: The transient nature of homelessness poses healthcare consistency challenges. Drawing from smallpox vaccine strategies, mobile clinics and community partnerships are effective. Building trust through tailored campaigns and community collaborations is crucial.
General Considerations: Skilled staff, robust data management, and inter-agency collaborations are essential for effective vaccination campaigns. Sufficient appropriations are required in order to build and maintain the missions of public health departments.
By addressing these populations' unique challenges, we can create an inclusive HIV-HCV coinfection strategy.
Future Medical Considerations:
The evolving nature of medical science presents new challenges and questions. The relationship between HIV and HCV may necessitate a tailored vaccine approach. Given experiences with COVID-19 and HBV vaccinations, how can we optimize the HCV vaccine for PLWH? Are there specific strategies to enhance its efficacy?
Public trust in health institutions remains fragile and highly politcized. How can we effectively communicate an HCV vaccine's importance and safety? How can we rebuild community trust?
Globally, ensuring the HCV vaccine's equitable access, especially in vulnerable populations with significant HIV-HCV co-infection risk, is a challenge. Can we learn from other vaccine distribution programs to strategize for HCV?
Urgent Considerations for HIV-HCV Coinfection's Future:
As we navigate the complexities of HIV-HCV coinfection, several pivotal questions arise, guiding researchers, policymakers, and healthcare professionals:
Vaccine Efficacy: Given varied vaccine responses in HIV patients, such as with COVID-19 and HBV, how can we optimize the HCV vaccine's effectiveness?
Access and Trust: How can we promote equal access, especially for vulnerable groups, and rebuild public trust?
Global Collaboration: How can we ensure global HCV vaccine access and which partnerships are essential?
Learning from History: Using insights from the U.S. Mpox outbreak, how can we better anticipate and manage health crises?
Policy Evolution: How can we swiftly incorporate evidence-based discoveries into health policies?
Actionable Recommendations for HIV-HCV Management:
To effectively combat HIV-HCV coinfection, we should consider:
Vaccination Strategies: Given varied responses among PLWH, explore frequent dosing, boosters, or double-dosing for the HCV vaccine, as seen with HBV and COVID-19.
Monitoring: Implement regular health assessments post-vaccination and periodic antibody and viral load tests.
Policy and Awareness: Prioritize coinfected individuals in vaccine rollouts, ensure accessibility, and launch awareness campaigns.
Collaborative Efforts: Foster interdisciplinary and global collaborations to holistically address HIV-HCV coinfection.
Addressing Current Deficiencies in Access: Despite curative therapies for HCV being readily accessible for more than decade, HCV remains a pressing public health concern in the United States. Effective vaccine distribution will hinge on addressing the challenges identified by these findings.
By strategically planning with these considerations in mind, we can create a comprehensive plan, prioritizing the well-being of those impacted by HIV-HCV co-infection.
Streamlining Vaccine Delivery and Building Trust in Healthcare
Efficient Vaccine Delivery: Successfully delivering vaccines for HIV-HCV co-infection requires more than just the vaccine. It's about a blend of skilled staff, efficient processes, and the right infrastructure:
Continuous Training: Ensure healthcare professionals are updated on the latest in vaccine administration for coinfections.
Resource Allocation: Balance routine healthcare with specialized vaccine campaigns, especially in resource-limited settings.
Infrastructure Upgrades: Enhance facilities, considering temperature-controlled storage and patient comfort, especially in remote areas.
Addressing Staffing Issues: Bridge the gap in healthcare professional shortages to ensure comprehensive care.
Workflow Efficiency: Use technology and process improvements for a seamless patient experience.
Community Health Worker Integration: Utilize their insights and community rapport to enhance healthcare delivery.
Feedback-Driven Improvements: Create a feedback-rich environment for continuous workflow enhancements.
Rebuilding Trust in Public Health: Trust is the bedrock of public health success, especially in the context of HIV-HCV coinfection:
Recognize Historical Mistrust: Address and make amends for past skepticism, especially among marginalized groups.
Combat Misinformation: Proactively counter myths about vaccines and transmission in the digital age.
Cultural Outreach: Use tailored messages and collaborate with community leaders for effective health drives.
Prioritize Transparency: Regularly update and demystify vaccine processes to foster trust.
Empathetic Engagement: Address vaccine hesitancy with understanding and compassion.
Collaborative Efforts: Partner with trusted community figures to amplify public health messages.
Feedback and Accountability: Implement public feedback mechanisms and act on them to reinforce trust.
In addressing HIV-HCV co-infection, both operational efficiency and trust-building are paramount. Together, they form the pillars of a comprehensive approach to public health challenges.
Conclusion:
Exploring the complexities of HIV-HCV coinfection reveals the depth of challenges and potential of modern healthcare. Each revelation, whether from studies on COVID-19, HBV, or HCV, not only highlight the gaps in our current understanding but also illuminates potential pathways forward. These insights should serve as guiding lights, directing our strategic development and interventions in the context of HIV-HCV co-infection.
However, our journey through this complex landscape is not solely guided by scientific discoveries. Central to our mission is a profound commitment to humanity and equity. It's a pledge to ensure that every individual, regardless of their background or circumstances, receives optimal care. From vulnerable groups, such as people experiencing homelessness or incarcerated persons, informed by lessons from the U.S. Mpox outbreak, to those in remote areas with limited healthcare access, our overarching goal remains steadfast: no one should be left behind.
By fostering collaboration across sectors, continuously updating our knowledge, ensuring investment in public health, and placing community engagement and trust at the forefront of our efforts, we can carve out a promising path. A trajectory that not only addresses the immediate challenges of HIV-HCV coinfection but also sets the stage for a healthier, more inclusive future for all affected individuals.
Upper-Payment Limits; Drug "Affordability" Boards Risk Medication Access
The opinion piece, authored by Jen Laws, CANN’s President & CEO, originally published in the September 2, 2023, print edition of the Denver Post. CANN will be hosting a free “PDAB 101” webinar for patients, advocates, and all public health stakeholders on November 1, 2023. Pre-registration is required. Register by clicking here.
To successfully combat the HIV epidemic and defeat other chronic conditions, patients must have uninterrupted access to the most effective medicines recommended by their doctors. As efforts to ensure patients can access their medicines are being defined in the public sphere, many state legislatures continue to advance policies and proposals focused on addressing patient affordability challenges.
However, many such actions fail to address high out-of-pocket costs and instead focus on lowering costs for other stakeholders within the health care system, like lowering costs and increasing profits for health insurers neglecting the patients they were intended to protect.
In Colorado and several other states across the country, lawmakers have empowered Prescription Drug Affordability Boards (PDABs) to address the rising costs that patients pay for prescription medicines. PDABs have the authority to select and review drug list prices and can recommend policies for drugs deemed "unaffordable." These list prices aren't something patients generally pay, rather we pay co-pays or are able to manage costs with patient assistance programs.
Despite this, one such policy being considered by the Colorado PDAB and similar boards in other states is an upper-payment limit (UPL). A UPL is a payment limit or ceiling that applies to all purchases and payments for certain high-cost drugs and does not necessarily translate into a "cost limit" for patients.
When UPLs are set, reimbursement rates are lowered for hospitals or clinics giving them less incentive to purchase specific drugs even though it may be the most effective medication to help a patient manage a chronic condition. When reimbursement rates are lowered through a UPL, it can also lead to barriers to biopharmaceutical companies investing in and supplying new innovative medicines to health facilities, making it difficult for doctors to prescribe treatments they think are best suited for their patients. While well intentioned, patients often bear the brunt of the challenges with such policies.
The impacts of the UPL process are only compounded when we consider the potential impact on the 340B Drug Pricing Program, a federal safety-net program that helps health facilities serve low-income and uninsured patients by offering them discounted drugs. Under the program, qualified clinics and other covered entities buy treatments at a discount to help treat vulnerable patients and get to keep the difference between the reimbursement rate and the discounted price leveraging those dollars to provide needy patients with medications and care they might not otherwise be able to afford.
Under a UPL, health facilities such as hospitals or clinics will receive lower reimbursements for prescribed treatments and therefore generate fewer dollars to support patients and the care we need to live and thrive. If the PDAB sets restrictive UPLs for drugs for chronic conditions like HIV, health facilities and the health professionals tasked with providing care will be faced with the decision to potentially stop prescribing these medicines and face having to cut support services that patients have come to rely on.
At a recent meeting of Colorado PDAB stakeholders, following the board's unanimous approval of the list of drugs eligible for an affordability review process, I voiced concerns about the approach to determining the value of lifesaving treatments for patients living with or at risk for HIV, hepatitis C (HCV), and other complex conditions. My concerns have only grown since, most recently, the state PDAB selected five drugs to undergo a formal affordability review including a treatment for HIV.
Many patients and other stakeholders have raised alarm to other drugs that are now subject to review to treat complex conditions such as psoriasis, arthritis, psoriatic arthritis, and cystic fibrosis. The implications of the Colorado drug "affordability" board's recent actions on patient access are grave and set a dangerous precedent. Ten states including Colorado have already established PDABs, and many others are following suit.
Those support services and continuity of care are critical to empower communities and improve the quality of life for people living with and managing conditions like HIV and hepatitis C. Despite the PDAB being "sold" to the public as a measure to improve patient experiences and access to care, the current model fails to prioritize patients at all.
Colorado is home to more than 13,000 people living with HIV and has been at the forefront of combating the disease. This year, state lawmakers advanced model legislation that protects patients' access to HIV prevention medication. However, the recent actions from the drug "affordability" board and short-sighted policies like the UPL process or mandatory generic switching could derail progress toward ending the HIV epidemic.
Price controls are, and will continue to be, a short-term, short-sighted "fix" with long-term consequences for patients living with chronic conditions. Policy efforts to address affordability must prioritize patient access and the ability for doctors to prescribe effective treatments. Colorado's PDAB, as it currently stands, falls short of that.
Alcohol Use Does Not Harm DAA Efficacy, Yet Payer Barriers Persist
In healthcare, the interplay between perceptions and policies can sometimes adversely affect the very individuals they intend to benefit. One such area of contention is the perceived impact of alcohol use on the effectiveness of treatments for hepatitis C Virus (HCV). A recent study, published in JAMA Network Open and spotlighted by MedPage Today, led by Christopher T. Rentsch, PhD, and co-authored by Emily J. Cartwright, MD, explored this relationship. Their findings were clear: alcohol use and alcohol use disorder (AUD) did not diminish the odds of achieving a sustained virologic response with Direct-Acting Antiviral (DAA) therapy for chronic HCV infection.
Yet, despite such evidence, certain clinicians still hesitate or even refuse to administer HCV therapy to patients who consume alcohol. Furthermore, some payers mandate alcohol abstinence as a precondition for reimbursing DAA therapy for HCV. This stance becomes even more alarming in light of the Center for Disease Control & Prevention's (CDC) recent data, which shows a staggering 129% surge in reported cases of acute hepatitis C since 2014. It's imperative that we prioritize evidence over misconceptions, especially when lives are at stake.
The NIH's Perspective
A study supported by the National Institutes of Health (NIH) echoes these findings, revealing that individuals with alcohol use disorder (AUD) are less likely to receive antiviral treatments for hepatitis C. Despite current guidelines recommending such treatment irrespective of alcohol use, the study, led by scientists at Yale University, found that those with AUD, even if they were currently abstinent, were less likely to receive curative DAA treatment for hepatitis C within one or three years of diagnosis compared to those without AUD. This treatment gap, attributed to stigma around substance use and concerns about treatment adherence, underscores the need to address these disparities, especially among those with AUD.
The Case for Change
The implications of these studies are clear: policies need revision. Evidence-based policies in healthcare are paramount. Denying HCV patients access to DAA therapy based on their alcohol consumption habits is not only unwarranted but also counterproductive. As the study's authors have highlighted, such restrictions could pose unnecessary barriers for patients and hinder efforts to eliminate HCV.
Both state-specific policies and national guidelines, like those from The American Association for the Study of Liver Diseases (AASLD), need to evolve in light of these findings. Healthcare providers, policymakers, and advocacy groups have a pivotal role in driving this change, ensuring that all HCV patients, irrespective of their alcohol consumption habits, have access to the best possible care.
Charting a Path Forward
The revelations from these studies underscore more than just the need for policy adjustments; they challenge our collective commitment to championing evidence-based healthcare. In an era where misinformation can easily cloud judgment, it's crucial that treatments for HCV are not just theoretically available but are genuinely accessible to all, regardless of their alcohol consumption habits.
The findings from both the NIH and JAMA studies don't merely point out gaps; they expose deep-rooted systemic issues. Current policies have not adequately addressed the needs of HCV patients, and there's a pressing need for more inclusive guidelines.
To transform this call to action into tangible progress, we must:
Reassess and Revise Existing Policies: Ensure that guidelines, especially those from influential bodies like AASLD, are updated in line with the latest scientific evidence, removing any unwarranted barriers related to alcohol consumption. As demonstrated by the efficacy of the Center for Health Law and Policy Innovation’s (CHLPI) work in assessing and breaking down barriers to curative DAAs in Medicaid programs, further work must be done to break these payer-based barriers to care in private and employer sponsored plans.
Strengthen Advocacy and Awareness: Engage with healthcare providers, policymakers, and patients to spread awareness about the non-impact of alcohol on DAA therapy's efficacy, countering prevailing misconceptions.
Promote Continuous Research and Dialogue: Encourage further studies and maintain an open dialogue with all stakeholders to continuously refine our understanding and approach to HCV treatment.
The conclusions drawn from these studies underscore the challenges and opportunities that lie ahead of us. As the research emphatically states, alcohol consumption should not be a barrier to HCV treatment. Such restrictions are discriminatory in nature and threaten efforts in the fight to eliminate HCV. With evidence-based policy decisions and unwavering dedication, we can eliminate the barriers and ensure access to curative HCV treatment.
Unveiling Disparities: OIG Report on HIV Care in Medicaid
A recent report by the U.S. Department of Health & Human Services’ (HHS) Office of Inspector General (OIG) unveiled a startling revelation: one in four Medicaid enrollees with HIV may not have received critical services in 2021. At a time when healthcare systems worldwide were grappling with the COVID-19 pandemic, these findings highlight the compounded struggles faced by People Living with HIV (PLWH) in accessing essential care.
Beyond safeguarding the health of PLWH, viral suppression plays a pivotal role in curbing HIV transmission, a critical metric in the public health effort to end HIV. In alignment with HHS guidelines, achieving consistent viral suppression necessitates three foundational elements: routine medical consultations, ongoing viral load assessments, and unwavering commitment to ART regimens.
Unveiling the Care Gaps
The OIG's analysis reveals pressing challenges within our system:
Out of the 265,493 Medicaid enrollees diagnosed with HIV, a startling 27% were missing evidence of receiving at least one of the pivotal services last year.
10% were missing evidence of medical visits, and 11% were missing evidence of an ART prescription, raising concerns around disease progression and higher incidence of AIDS diagnosis, increased risk of transmitting the virus, and developing antiviral resistance.
The most pronounced gap? Viral load tests. A staggering 23% of enrollees lacked evidence of even a single viral load test in 2021. This absence not only impedes clinicians from making informed decisions but also hampers our collective ability to monitor and respond to the evolving nature of the HIV epidemic.
Perhaps most concerning is the fact that more than 11,000 of these enrollees didn't have evidence of availing any of the three critical services. This is not just a statistic; it's a reflection of real individuals, facing amplified health risks due to system inadequacies.
Jen Laws, CANN's President and CEO, poignantly remarked, "Medicaid represents the greatest public program coverage of PLWH. It also represents the greatest public program coverage of people at risk of acquiring HIV. Medicaid compliance and efficacy is critical to Ending the HIV Epidemic and this report has identified gaps where states have failed to meet their obligations to Medicaid beneficiaries and where CMS has failed to ensure compliance. We must do better if we are going to reach our public health goals."
The report underscores stark disparities in care access between Medicaid-only and dual-eligible enrollees (those with both Medicaid and Medicare). Specifically, Medicaid-only enrollees were three times more likely to lack evidence of any of the three critical services compared to dual-eligible enrollees, with 6% of Medicaid-only enrollees missing out, as opposed to just 2% of the dual-eligible group. Such disparities might stem from various factors, including Medicare's higher fee-for-service rates and the observed long-standing adherence patterns among older adults who have had HIV for prolonged periods.
State-wide Disparities: A Complex Landscape
The disparities in HIV care access across states offer both a grim reality check and a clarion call for systemic reform. Drawing from the OIG report, certain states, notably Arizona, Arkansas, the District of Columbia, and Utah, have alarmingly high proportions of Medicaid-only enrollees without evidence of at least one of the three critical services. For instance, Utah stands out with a staggering 87% of such enrollees missing out on essential care. These aren't just numbers; they represent real individuals grappling with a system that's failing them.
Conversely, some states demonstrate better compliance and efficacy in delivering HIV care, which suggests potential models or strategies that could be emulated across the board. However, the stark variability across states points to the undeniable influence of state-specific policies, the quality of local HIV care infrastructures, and broader challenges associated with healthcare access.
State agencies clearly have much work ahead. These disparities not only indicate potential inefficiencies or gaps in policy implementation but also suggest a pressing need for introspection and reform at the state level. Coupled with challenges in the broader Medicaid system, there's a compelling case for a comprehensive overhaul.
The onus is on both state agencies and the Centers for Medicare & Medicaid Services (CMS) to rise to the occasion. The disparities, as evidenced in the report, necessitate a deep dive to understand the underlying causes. Is it a matter of policy misalignment, funding constraints, administrative challenges, or a combination thereof?
For instance, the significant variation in care access among dual-eligible enrollees, ranging from 9% to 53% across states, speaks volumes about the discrepancies in policy implementation and oversight. It's vital to pinpoint these issues, develop tailored interventions, and ensure that every Medicaid enrollee, irrespective of their state, receives the essential services they need.
The Pandemic's Shadow
The COVID-19 pandemic exerted immense pressure on healthcare systems, with profound implications for HIV care. The OIG report highlights the challenges faced by Medicaid enrollees with HIV during the COVID-19 pandemic. A significant finding from the report is the notable deficiency in viral load tests among these enrollees. This underscores the need for healthcare systems to effectively manage and prioritize essential services, even amidst broader healthcare crises. The report serves as a reminder of the importance of consistent and uninterrupted care for conditions like HIV, even when healthcare systems face external pressures.
Steering Towards a Brighter Future
Addressing the glaring disparities laid out in the OIG report goes beyond mere policy recalibration; it challenges our collective resolve to uphold equitable healthcare. In the shadow of the pandemic's aftermath, it's imperative that essential services for PLWH are not just nominally available but are genuinely accessible.
The OIG report's findings don't merely spotlight discrepancies; they highlight systemic lapses. States have fallen short in fulfilling their obligations to Medicaid beneficiaries, and the CMS has not adequately ensured compliance.
To translate this call to action into meaningful change, it's essential to:
Strengthen state-level accountability frameworks to ensure Medicaid obligations are met comprehensively, particularly among states utilizing Managed Care Organizations – ensuring those contracted for-profit insurance companies are meeting their contractual obligations to states. The tools for accountability already exist within these contracts, they merely must be used.
Bolster CMS oversight mechanisms, driving proactive interventions that hold states to account and rectify compliance lapses.
Engage in continuous dialogue with stakeholders, including healthcare providers and beneficiaries, to identify and remedy bottlenecks in care access.
The findings from the OIG report serve as a stark reminder of the work ahead. As Jen Laws aptly states, "We must do better." HIV advocates should consider assessing our engagement with their state Medicaid programs and look for opportunities to act, akin to our engagement Ryan White programs. With concerted efforts, policy reforms, and collective commitment, we can bridge the gaps and ensure comprehensive care for all PLWH.
340B Hypocrisy: The Inconvenient Truth Behind Why We Need to Reform This Vital Safety Net Program
Brandon Macsata is the CEO of ADAP Advocacy. Jen Laws is the CEO of Community Access National Network.
The 340B Drug Pricing Program (“340B”) is probably one of the most transformative public health programs providing lifesaving supports and services to people living with HIV in the United States, second only to the Ryan White HIV/AIDS Program (“RWHAP”). As such, rigorous debate about the future of the program is not only healthy, but it is also paramount to its success. As patients (and patient advocates), it is our responsibility to demand accountability, transparency, and stability. There is universal agreement about the vital role 340B plays in improving access to healthcare. But for many – including ADAP Advocacy and the Community Access National Network – we contend that the program could be doing more…and better! The focus of the program should be on the patients, and not the Covered Entities, medical or service providers, or any other business enterprises making lots of money off it. That is the inconvenient truth behind why we need to reform this vital safety net program.
Section 340B of the Public Health Service Act (PHSA) is a Drug Pricing Program established by the Veterans Health Care Act of 1992. That year, Congress struck a deal with pharmaceutical manufacturers to expand access to care and medication for more patients; if pharmaceutical manufacturers wanted to be included in Medicaid’s coverage, then they’d have to offer their products to outpatient entities serving low-income patients at a discount. The idea was brilliantly simple. Drug manufacturers could have a guaranteed income from participation in the Medicaid program and Covered Entities could have guaranteed access to discounted medications. Congress set-up a payment system by way of rebates and discounts affording certain healthcare providers a way to fund much needed care to patients who could not otherwise afford it.
“…to stretch scarce Federal resources as far as possible, reaching more eligible patients and providing more comprehensive services.” H.R. Rep. No. 102-384(II), at 12 (1992)
THAT is the legislative intent behind 340B. THAT is where some of us want to return 340B’s focus. THAT is why reform is coming!
Ironically, critics of the 340B reform movement – often motivated by self-preservation and protecting their ever-expanding budget and geographic footprint – are quick to attack the idea of the need for reforms. Sadly, they’re also quick to turn their criticism into personal attacks, including questioning the intentions, morals, and character of the people supporting reform. They charge, using Inspector Clouseau “gotcha” style rhetoric, that we’re in the “pockets” of the drug manufacturers because we accept their money to help with our patient advocacy and education (yet there is no “gotcha”, since this information is quite publicly available on our websites, annual tax returns, Guidestar, as well as frequent public commentary).
Isn’t it funny how the “gotcha” mentality cannot accept the obvious, that maybe our interests align with the drug manufacturers because it is in the best interest of patients. Drug manufacturers make products patients want and need. Ensuring funding flows in a way that expands patient access to medications does indeed benefit both patients and the drug manufacturers. It should be noted, this criticism tends to also neglect mentioning the interests of the entities challenging reform: anti-competitive consolidation among hospitals and pharmacies (leaving whole areas without services), increasing profits, paying for salaries unrelated to healthcare, and increasing administrative salaries are all excellent examples of why we’re left asking “Who is actually benefiting from this program?”
The truth of the matter is, aside from a growing list of patients, patient advocacy organizations, and drug manufacturers, there is a growing chorus calling for reform. Academia wants it (NEJM, Penn LDI, USC Schaeffer), economists want it (Nikpay, Gracia), national trade associations want it (NACHC, NTU), policy think tanks want it (CMPI, NAN), and even multiple news media outlets are suggesting it (Forbes, NYT, WSJ). Local activists are also increasingly fed-up with what they’re witnessing (Dinkins, Feldman, Winstead).
Dr. Diane Nugent, Founder & Medical Director of the Center for Inherited Blood Disorders, recently noted an opinion piece in the Times of San Diego, “A September 2022 analysis by the Community Oncology Alliance revealed that some hospitals participating in 340B price leading oncology medications nearly five times more than the price they paid. Another study found that hospital systems charge an average of 86% more than private clinics for cancer drug infusions.”
But speaking of deep pockets, isn’t it also an inconvenient truth that the very folks fighting reform, and fighting improving the program so patients can benefit more directly from it, are the same folks financed by big hospital systems, and mega service providers abusing 340B intent?
A question often asked by advocates learning about 340B: “So, exactly how much money are we talking about here?”
Well, we don’t really know…sort of. For Federal Grantees covered under 340B, their grant contracts require accounting of 340B rebates as part of their programmatic revenues. Those revenues are required to be re-invested in the program, which generated the income. This level of transparency is pretty much a “gold standard” that other Covered Entities (less maybe hemophiliac centers) in the 340B space are required to meet. That’s part of why we, and other advocates, are calling on minimum reporting requirements for hospitals, contract pharmacies, and pharmacy benefit managers (insurers covering medications) to begin providing some data. Clearing up the murkiness, if you will. What we do know is drug manufacturers reported more than $100 BILLION in 340B-related sales last year.
That’s concerning especially because “charity care” is declining and medical debt is a growing issue for more and more patients and their families. The Affordable Care Act mandated “charity care”, or “financial assistance”, to be offered by non-profit hospitals seeking to qualify as 340B entities but did not place any definitions behind the mandate, including any “floor” of how much charity care a hospital has to offer.
Now, in all rhetoric opposing any type of transparency in 340B, hospitals tend to conflate their “uncompensated care” and “unreimbursed care” or “off-sets” for public health programs – these don’t necessarily reflect any “charity” being provided to patients. These things should be separated when considering what benefit hospitals provide a community. And under that lens, things get kind of ugly with far too many of the 340B hospitals reporting providing less than 1% of their operating costs as charity. When reviewing how much hospitals write off in bad debt, or going after patients who can’t afford care, often far exceeding those charity care levels, we’re left asking if the “non-profit” designation is really a declaration of concentrating “profits” by way of salaries to top executives rather than formal shareholders?
That bad debt shows up for patients as medical debt. And we need to be very specific here: according to the Urban Institute, some 72% of patients with medical debt owe some or all of that debt to hospitals. Meaning, what we call medical debt is really hospital debt. The situation is unarguably bad. This year alone the Los Angeles County Office of Public Health issued a report outlining for policymakers the role and responsibility hospitals have in driving medical debt and how increasing charity care might stem this problem.
As patients, and frankly as patient advocates who represent thousands like us, medical debt isn’t an issue that can be swept under the carpet. Entire communities avoid necessary care to protect their financial interests. We’ve personally watched our friends open GoFundMe accounts to cover medical expenses. We’ve helped our loved one’s cover food and light bills to not miss a medical bill. We also well recognize how negative credit reporting from medical debt can hurt people from getting rental housing or a car loan, or even simple necessities. And when thinking about how much we don’t know about what’s behind that $100 billion price tag, the fact that patients face these concerns on the regular is pretty obscene.
We do know there are plenty of good actors in the 340B space. Particularly, Federal Grantee Covered Entities, like Ryan White Clinics and AIDS Drug Assistance Programs (ADAPs). And we know they’re generally great actors because of that transparency in reporting and the oversight offered by their grant contracts. Ultimately, we’re not necessarily asking for a whole lot more than that for literally everyone else who stands to make a buck in the chain between drug manufacturers and patients. Indeed, that trust on Federal Grantees, particularly Ryan White Clinics and ADAPs, is part of why drug manufacturers restricting 340B sales held a carve out for these Federal Grantees. (To be fair and without much public fanfare, years ago, we – as in ADAP Advocacy and CANN – helped to negotiate these carve-outs as part of our advocacy. Our relationship with drug manufacturers isn’t a one-way street as detractors might try and sell you on.
$100 billion is a lot of money! Is it too much to ask, “Why aren’t patients benefiting more directly from this ever-growing healthcare program?” Facts show that 340B revenues are soaring year after year, yet against the grim backdrop of consistently declining charity care in the impoverished communities needing the most help. To make matters worse, rising medical debt is crushing families. Patients deserve better. People living with HIV who depend on the RWHAP and 340B deserve better! And that is why we need reform.
Read our policy reform suggestions here.
Prescription Drug Advisory Boards: Who is Impacted and How to get Involved
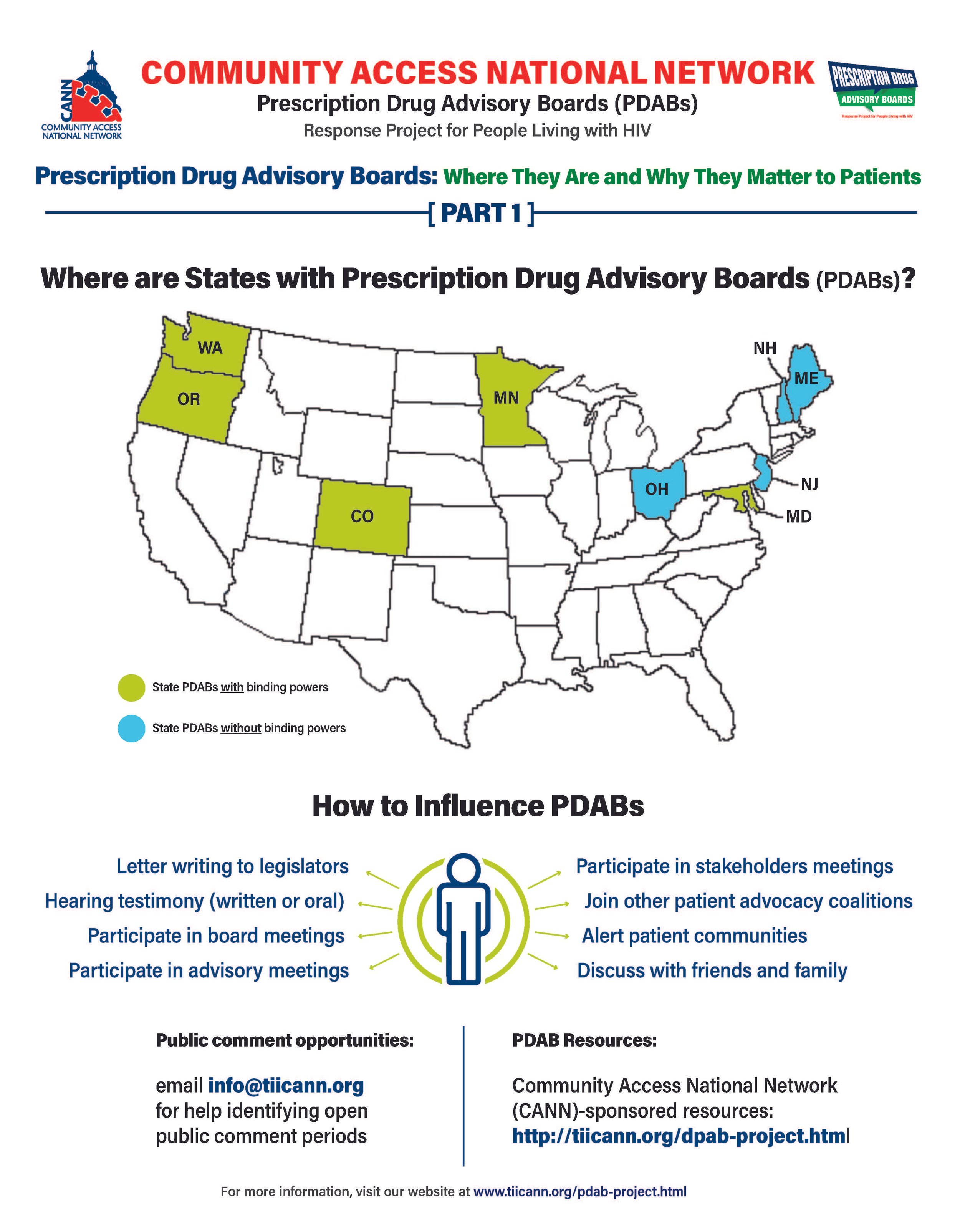
The prescription drug advisory board (PDAB) train keeps chugging along. Presently, there are nine (9) states that had, have or are in the process of enacting PDAB legislation: Washington, Oregon, Colorado, Michigan, Minnesota, New Jersey, New Hampshire, Maryland, and Maine. Ohio, it would seem, has abandoned their PDAB efforts. Their geographical variance reflects the diversity of their structures. Some of the boards have five members, and some have seven. While all are appointed by the governor, they differ regarding which departments they are associated with. For example, Colorado’s is associated with the Division of Insurance, and Oregon’s is associated with the Department of Consumer and Business Services.
The assortment of structure does not stop at department association. The number of drugs to be selected annually for review also varies, such as Colorado with five and Oregon with nine. Even the number of advisory council members lacks consistency. The New Jersey DPAB advisory council has twenty-seven (27) members, while Colorado’s has fifteen (15). Inconsistency in structure means inconsistency in operations. Thus, the help or harm patients ultimately receive will vary drastically from state to state. The most important differences are the powers bestowed upon the various DPABs. In addition to shaping many policy recommendations, five (5) currently have the ability to enact binding upper payment limit (UPL) settings: Washington, Oregon, Colorado, Maryland, and Minnesota.
An upper payment limit sets a maximum for all purchases and payments for expensive drugs. By setting UPLs for high-cost medications, improved ability to finance treatment equals greater access to high-cost medicines. A UPL sets a ceiling on what a payor may reimburse for a drug, including public health plans, like Medicaid.
Patients, advocates, caregivers, and providers are concerned about PDABs because the outcomes of theory versus practice can have dire consequences. Theoretically, PDABs should reduce what patients spend out of pocket for medications and lower government prescription drug expenditures. However, the varied ways different PDABs are set to operate could jeopardize goals. Focusing on lowering reimbursement rates could affect the funds used as a lifeline by organizations benefiting from the 340B pricing program even while not meaningfully reducing patient out-of-pocket costs. If reimbursement limits are set too low, those entities will have drastic reductions in the funding they use for services for the vulnerable populations they serve. UPLs could ultimately increase patients' financial burden if payers increase cost-sharing and change formulary tiers to offset profit loss from pricing changes or institute utilization management practices like step-therapy or prior authorization. Increasing patient administrative burden necessarily decreases access to medication. When patients are made to spend more time arguing for the medication they and their provider have determined to be the best suited for them, rather than simply being able to access the medication, the more likely patients are to have to miss work to fight for the medication they need or make multiple pharmacy trips – or suffer the health and financial consequences of having to “fail” a different medication first. PDAB changes could affect provider reimbursement, which could be lowered with pervasive pricing changes. Decreased provider reimbursement could result in additional costs being passed onto patients or, in the situation of 340B, safety-net providers, reduce available funding for support services patients have come to rely upon.
The divergent factors that different PDABs use for decision-making are of concern as well. It is not enough to just look at the list price of drugs and the number of people using them. For example, some worthwhile criteria for consideration of affordability challenges codified in Oregon’s PDAB legislation are: “Whether the prescription drug has led to health inequities in communities of color… The impact on patient access to the drug considering standard prescription drug benefit designs in health insurance plans offered…The relative financial impacts to health, medical or social services costs as can be quantified and compared to the costs of existing therapeutic alternatives…”. But few of these PDABs consider payer-related issues like limited in-network pharmacies, discriminatory reimbursement, patient steering mechanisms, or frequency of utilization management as hindrances to patients getting our medications.
Effectively seeking and considering input from patients, caregivers, and frontline healthcare providers is also of concern. The legislation of various DPABs specifies the conflicts of interest that board members cannot have and must disclose. Some even have appointed alternates to allow board members to recuse themselves from making decisions on drugs with which they have financial and ethical conflicts. However, most of the advisory boards are providers, government, and otherwise industry-related. The board members are even required to have advanced degrees and experience in health economics, administration, and more. The majority of the discourse is not weighted towards the patient and our advocates. Few, if any, specific active outreach measures when it comes to seeking patient input. For example, the Ryan White HIV/AIDS Program requires patient and community engagement outlets in planning activities. But no PDAB legislation, to our knowledge, requires PDABs to engage with these established patient-oriented consortia. We know well in HIV that expecting already burdened patients often struggle to meet limit engagement opportunities from government boards – we know the very best practices are going to patients, rather than expecting patients to come to these boards. Beyond these limited engagement opportunities and failure to reach out to spaces where patients are already engaged, some states have exceptionally short periods in which to gather these inputs.
However, depending on the individual state’s DPAB structure, there is an opportunity for patients, caregivers, and organizations to give input through public comment periods and particular meetings aimed at stakeholder engagement. For states considering PDAB legislation, like Michigan, patients can and should engage in the legislative process. One place to keep abreast of different state’s PDAB activities is the Community Access National Network’s PDAB microsite. The microsite has an interactive map where you can access various states’ PDAB sites as they are created. States with fully formed PDABs have sites that display their scheduled meetings, previous decisions made, agendas for future sessions, and, most notably, details of the process for the public to provide input. Most of the meetings are open to the public, with the public invited to provide oral public comment or to submit written comments. Attending meetings and speaking directly to the boards is a way to have board members and others hear directly from those who will be affected by their decisions. Written public comment is also essential, especially from community patient advocacy organizations. Some DPABS also provide access to virtual meetings where stakeholders can provide feedback and input.
Medicare has six protected drug classes: anticonvulsants, antidepressants, antineoplastics, antipsychotics, antiretrovirals, and immunosuppressants. This means that Medicare Part D formularies must include them but that protection exists because we know how important these medications are. Antiretrovirals and oncology medications are a part of that list because adversely affecting the mechanisms of access to those drug classes is life-threatening to those who need them. It is imperative that continued scrutiny be placed upon DPABs to ensure that their benefits are patient-focused, like reducing administrative burden and barriers to care, rather than a mask that ultimately benefits payers by increasing their profits.
Underprepared: Opioid Settlement Dollars are Coming
The opioid epidemic has ravaged communities across the U.S., resulting in significant settlements from the pharmaceutical industry. However, the allocation of these funds, likely to exceed $50 billion, raises concerns about potential mismanagement.
Past public health crises have led to significant settlements. The 1998 Tobacco Master Settlement Agreement, for instance, was heralded as a landmark deal. Major tobacco companies agreed to pay billions to 46 U.S. states, funds that were ostensibly earmarked for anti-smoking campaigns and health programs. Yet, as research from RAND later revealed, a significant portion of these funds were diverted to unrelated projects. The promise of a healthier future was overshadowed by the allure of immediate fiscal relief, a misstep that has had lasting implications and begs the question. Will the opioid settlement reach the same result or have states learned their lesson?
Recent Concerns
Probably not, as the misuse of settlement funds remains a concern:
•COVID Funds Misdirection: In a move that sparked controversy, some states opted to use COVID relief funds for prisons, diverting resources from pandemic relief efforts. This decision underscores the tension between immediate fiscal needs and long-term public health goals.
•Mendocino County's Opioid Funds Dilemma: In a decision that drew sharp criticism, especially from those directly affected by the opioid crisis, Mendocino County used over $63,000 of opioid settlement funds to address a budget shortfall, despite having the highest rate of overdose deaths in California.
•New York's Opioid Funds Controversy: Raising eyebrows and questions about the state's priorities, funds intended for opioid crisis mitigation in New York were instead used for overtime expenses related to narcotics investigations.
The Current Landscape
While the anticipated $50 billion from opioid lawsuits offers hope, the lack of standardization and oversight in fund distribution is concerning. The primary objective of these funds is to bolster prevention, treatment, and recovery infrastructure, but it is feared that the absence of clear guidelines and reporting mechanisms will lead to misallocation and abuse. Only 12 states have committed to detailed reporting, emphasizing the need for transparency.
The Profit-Driven Rehab Industry's Ethical Crisis
Challenges posed by the profit-driven rehab industry in the U.S. include aggressive sales techniques, overcharging, and substandard care. The system often pushes vulnerable individuals into treatments that may not be in their best interest. The Affordable Care Act, while praised for mandating private insurance programs to cover addiction treatments, inadvertently led to a surge in for-profit rehab clinics, some of which prioritize profit over patient care, further emphasizing the need for rigorous oversight and quality standards. Few state officials are familiar with these market and health landscape dynamics, meaning few officials are ready to offer the necessary oversight of these dollars and the programs they’ll be going to support. That includes drug court programs.
A recent investigation by Spotlight PA highlighted the lax oversight of addiction treatment facilities in Pennsylvania. The Department of Drug and Alcohol Programs (DDAP) in Pennsylvania has been criticized for allowing providers to continue operating despite repeated violations and harm to clients. The tragic story of Adam Kalinowski, who died less than 24 hours after entering a treatment center known as Addiction Specialists, Inc. (ASI), serves as a poignant example. ASI had a history of violating state rules, and a wrongful death suit against them resulted in a judgment of over $1.6 million in damages.
Drug Courts
In response to surging drug-related criminal cases, drug courts have emerged as a solution, offering offenders a chance at rehabilitation instead of incarceration. However, there are serious vulnerabilities. Recent revelations in Louisiana provide an example of how lax federal oversight of the Department of Health and Human Services (HHS) grants funding of drug courts have lead to corruption, kickbacks, and questionable practices within these drug court systems and the treatment centers they refer defendants to.
In Lafayette, Louisiana, a mysterious $3 million appropriation for a substance abuse rehab facility became the epicenter of controversy. In the previous year, while the state Senate was formulating the state budget, an unusual amendment was introduced, directing $3 million to a governmental health organization in Lafayette for a 70-bed addiction treatment center. It was later revealed that three businessmen, Mark Fontenot, Jeff Richardson, and Leonard Franques, were advocating for this funding to establish a substance abuse rehabilitation facility in Lafayette. Franques is currently at the heart of an expanding bribery investigation that has implicated officials from the 15th Judicial District Attorney’s office in Lafayette, among others. The scheme involved DA Office kickbacks for steering pretrial diversion defendants to four businesses, including Lake Wellness Center, Franques' outpatient rehab facility.
The scandal in Lafayette highlights the intricate web of connections and potential conflicts of interest surrounding substance abuse rehab facilities, the justice system, and state legislatures who will be in charge of setting appropriations for these historic opioid settlement funds. Harm reduction and Justice advocates will need to work closely together in order to push for necessary “watch dog” activities and opportunities in these referral systems.
The Crisis of Medication Assisted Treatment Access for Minors
The rise of fentanyl has dramatically altered the landscape of opioid addiction. Teenagers are developing severe dependencies at an alarming rate, transitioning rapidly from experimentation to intense dependence. This swift onset of addiction underscores the urgent need for effective treatments tailored to this age group.
Despite the proven efficacy of buprenorphine, considered the gold standard for treating opioid use disorder, less than a quarter of residential treatment centers for adolescents offer it. This lack of access is deeply concerning, especially given the sharp rise in overdose deaths among teenagers, exacerbated by the proliferation of fentanyl.
Several barriers hinder the provision of MAT to minors:
• Philosophical Objections: Some facilities object to medications like buprenorphine on philosophical grounds, despite its proven efficacy.
• Lack of Expertise: Many treatment centers lack the necessary expertise to treat adolescents with MATs.
• Stigma: The stigma associated with MATs, especially among teenagers, poses a significant barrier. If teenagers feel marginalized for taking medication, they might avoid it.
• Systemic Barriers: A shortage of certified providers and underfunded facilities highlight the systemic challenges that need to be addressed to tackle the opioid crisis effectively.
The lack of MAT access for minors raises concerns about the allocation of opioid settlement funds. The funds are intended to address the opioid crisis head-on. If they aren't used to ensure access to MAT for all, including minors, public trust in the system could erode. Furthermore, without access to effective treatments and education, teenagers are at a higher risk of overdose and death. Addressing the barriers to MAT access for teenagers is crucial to ensure that the funds are used effectively and that this vulnerable population receives the care they desperately need.
The Role of the Department of Health and Human Services
The HHS plays a pivotal role in shaping the nation's response to the opioid epidemic. It oversees fund allocation, issues grants to incentivize particular programming, and sets care standards. Ensuring these standards are stringent and patient-centric is vital. State health departments face challenges, including staffing shortages, which can impact fund management.
State Health Department Challenges
State health departments, such as the North Carolina Department of Health and Human Services (NCDHHS), play a crucial role in addressing the opioid crisis at the local level. However, these departments face significant challenges, including staffing shortages and budget constraints. For instance, the NCDHHS has grappled with a 28% vacancy rate, which has doubled since the onset of COVID. Such staffing shortages can severely hamper the department's ability to manage and allocate funds effectively. These challenges have direct implications for local initiatives, such as the Queen City Harm Reduction's housing pilot program, which faced delays due to funding issues.
Lack of Guidance on Contract Quality with Local Drug Courts
While the HHS provides oversight and sets standards for care, there has been a notable lack of guidance on increasing contract quality between local drug courts, private and publicly funded managed care programs, and providers. Given the potential for conflicts of interest and corruption within the drug court system, as evidenced by the Lafayette bribery scandal, this lack of guidance is concerning. Ensuring transparency, accountability, and quality in contracts is a key factor that will ensure opioid settlement funds are effectively used at every level.
Conclusion and Call to Action
The opioid epidemic presents a monumental public health challenge. The opioid settlement funds offer a unique opportunity to address these interlinked crises. However, without stringent oversight and a clear roadmap, there's a risk that these funds might not be used to their maximum potential.
The rapid allocation of funds without proper oversight is a recipe for disaster. It's crucial to ensure that these funds are channeled into comprehensive programs that not only address OUD but also the associated risks of HIV and HCV infections.
The opioid epidemic and the associated settlement funds present both an opportunity and a challenge. Proper oversight is essential to ensure these funds are used effectively. Advocacy groups, community leaders, and stakeholders must rally together to push for rigorous HHS contract quality standards, ensuring transparency and accountability.
CDC Publishes New Medical Monitoring Data
On August 22nd, the Centers for Disease Control and Prevention (CDC) published new data from the Medical Monitoring Project (MMP). The MMP is an annual, national survey sample evaluating certain behavioral and clinical characteristics of people living with HIV (PLWH). Particularly, the report now includes certain quality of life metrics and stigma related factors as suggested in the National HIV/AIDS Strategy (NHAS). Data was reported from the 2021 cycle, meaning collected from June of 2021 through May of 2022.These measures relating to stigma and quality of life offer insight as to the experiences of PLWH and potential barriers to care – ranging from housing and food insecurity to mental and emotional health.
The findings were a bit of a mixed bag. NHAS suggests an overall health rating of “good” or “better” among participants should reach 95% or better to consider this metric goal achieved. However, the 021 cycle saw a drop in overall health rating of “good” or “better” from 72% in 2020 to 69% in 2021, the lowest since 2018.
Similarly, the measure of “unmet needs” for mental health services increased from 21% in 2020 to 28% in 2021. This is also the highest rate of unmet mental health services needs since 2017. NASH sets the goal for this metric at 12%. In order to address these needs, federal and state funding designs and initiatives should reflect this priority. Among them, regulatory enforcement on mental health parity among insurance networks and reimbursement rates, especially in Medicaid programs, and public policy efforts aimed at those issues which affect mental health and overall health (like housing and food security and stigma via strengthening of anti-discrimination laws and incentivizing best practices in areas of life affecting people living with HIV such as child welfare programs, education programs, and among family courts). Why?
Among participants, 9% reported experiencing symptoms of major depression within two weeks of taking the survey and 7% reported symptoms of “other depression” in the two weeks prior to the interview. Similarly, 5% of participants reported “mild anxiety” in the two weeks previous to the interview and 8% reported “moderate anxiety” with the same percentage reporting “severe anxiety” during their interviews. In addition to these, 29% of participants reported experiencing intimate partner violence at some point in their lives, with 5% reporting intimate partner violence in the last 12 months and 17% reporting having experienced rape* in their lifetime and 1% within the last 12 months.
The neutral news is that the percentage of PLWH in an “unstable housing” situation has stayed steady from 2020 to 2021 at 17%. Given the economic upheaval of 2020 and continuing through 2021 and 2022, maintaining a rate of housing security is a notable achievement for our housing programs. However, NHAS sets the metric goal at 11%, meaning there’s still plenty of room for improvement in ensuring PLWH are adequately housed. Along these same lines, “food insecurity” also maintained its 2020 rate at 16%. Layered onto the human rights and sheer necessity of food access, many antiretroviral tablet regimens and medications used to treat co-occurring conditions require food in order to be properly absorbed. NHAS sets the goal for this metric also at 11%. If PLWH do not have sufficient, secure sources of nutrition and housing, all other care will fall by the way side. 8% and 38% of participants surveyed reported experiencing homelessness or having their household live in poverty, respectively. 16% of survey participants reported experiencing hunger or food insecurity.
Unemployment among PLWH dropped in 2021 to 15%, just higher than the 14% in 2019. 2020, for obvious reasons related to the emergency phase of the COVID-19 pandemic, saw a dramatic spike in unemployment among PLWH to 18% - far higher than the national average of 13% through 2020. Employment often offers PLWH not only a secure source of income, it also often offers insurance coverage and a pathway to security in housing and food. Strengthening the workforce is absolutely a necessity in achieving NHAS’s goal of at most 8% unemployment among PLWH. 39% of participants surveyed reported being unemployed or lacking an ability to work.
Lastly, and likely the most heartbreaking data from the survey, trends in stigma have reversed course from a 3% reduction in 2020 at 28%, increasing to 29% in 2021. Ensuring PLWH are not stigmatized when in care, at work, seeking housing or education, or when faced with those in positions of authority in other areas of life is critically important to ensuring we reduce experiences with harmful stereotypes. We can start by defining stigma in concrete terms and advocating for policies addressing those aspects of life for PLWH. While the NHAS sets the goal for this metric at 16%, even that’s still an unacceptably high level of stigma for the 1.2 million people living with HIV in the United States.
Payors participants utilized was highest for those enrolled in Ryan White coverage at 47%, Medicaid covered 43%, private plans covered 42%, 29% were covered by Medicare, and 9% reported being uninsured. 66% of participants reported viral suppression at their most recent clinical test with 62% reporting “sustained” viral suppression. 95% received outpatient care, 71% reported being retained in care, 81% reported they did not miss their HIV-related provider appointments, and 80% reported being prescribed antiretroviral medications. Reasons ever missing doses were reported as follows: 65% simply forgot to take their medications at some point, 42% reported a change in routine disrupted their medication cycle, 40% reported falling asleep early or oversleeping as a cause to miss a dose, 17% reported feeling depressed or overwhelmed, and – not shockingly but it really should be – 16% had a problem getting their HIV medication prescription filled or refilled.
MMP sites are located in 16 states and 6 separate funding jurisdictions. Participants gender demographics were identified as 23% cisgender women, 75% cisgender men, and 2% “transgender” with no differentiation among identity or sex assigned at birth for transgender participants. 55% of participants were older than 50, 20% were between the ages of 40-49, 18% were between the ages of 30-39, 7% were among those aged 18-29, and no participants younger than 18 were included. 43% reported their sexual identity as heterosexual or straight and 43% reported identifying as lesbian or gay, 10% reported identifying as bisexual, and 4% reported identifying as “another sexual orientation”. Lastly, the racial make up of the cohort was reported as follows: 41% African American/Black, 28% White, 24% Hispanic/Latino, 5% multiracial, 1% Asian, less than 1% American Indian/Native Alaskan. While the report was analyzed under a lens of accounting for certain skewed demographic participation, the limitation of experience should be noted.
Medicaid Unwinding is Going Terribly: By the Numbers
April 1st came quickly in the state of Florida this year. It was the day that began the unwinding of the state’s continuous coverage of Medicaid as part of the federal government’s COVID-19 pandemic response. Not every state began at the same time and the Biden Administration had issued guidance to states that amounted to “take your time, please. Don’t rush this.” But Florida, among other states, wasn’t so interested in taking advantage of waivers the Centers for Medicare and Medicaid Services (CMS) offered, to help ease the speed in which states might end up disenrolling patients thanks, in some part, to automated redeterminations. Now, these redeterminations are supposed to cross reference available tax and income data in order to tap the breaks some when it comes to financial eligibility of a beneficiary. How efficiently that’s happening, well… here’s some updates to paint the picture.
In June 2023, Kaiser Family Foundation (KFF) issued an analysis that between 8 million and 24 million beneficiaries would potentially lose coverage during the unwinding period (about 18 months), largely dependent on how states handled the redetermination process. As of their effort, KFF’s tracker estimates about 5.5 million beneficiaries have been disenrolled and about 8.9 million have retained their Medicaid coverage in the 45 states (and D.C.) providing data. Of those 5.5 million, about 1.15 million are children or about 43% in the states providing data with age breakouts (that is, only about 15 states). 74% of all disenrollments are for “procedural” reasons, rather than any determination of actual eligibility.
Florida is one of just two states to decline taking any of the 17 types of waivers CMS offered states to ease the unwinding process. Montana is the other. Every state received letters earlier in the month outlining CMS’ concerns regarding three core areas of concern, which are situated in requirements under federal law and regulations; slow application processing, long call center wait times, and high rates of people losing coverage due to paperwork issues. While 36 states got flagged for being weak on at least one criterion, five states were behind on all three: Alaska, New Mexico, Rhode Island and – you guessed it – Florida and Montana. 16 states were cited for long call center wait times. 16 states were cited for having not processed applications within a 45-day window.
Rhode Island is the only state that issued a statement to Politico in response to the letters saying that it was delaying ending coverage for families and children until the beginning of 2024.
Despite Florida having a relatively low disenrollment rate of 31% (Texas’ disenrollment rate is 72% and the highest in the country, 11 states have a disenrollment rate above 50%), families in the state have had enough. Representing two specific families and the families like them across the state, the Florida Health Justice Project and the National Health Law Program sued the state of Florida on August 22nd. One family’s toddler missed weeks of their cystic fibrosis medication after being cut off and the other had their one-year-old miss a routine vaccination after their provider notified them the appointment was canceled due to lack of coverage. Both families are alleging they were not properly notified or given the chance to appeal the decision before termination of coverage – both are federal requirements.
This is the type of claim the Biden Administration is going to be itching at intervening on because it validates the concerns they’re been broadcasting about the unwinding process.
With all that comes the very real risk of a judiciary changed by the previous administration taking aim at the federal government’s powers and programs targeted at helping poorer communities. Advocates exercising legal strategy should watch these developments closely and engage with their state and federal legislators to ensure the outcomes of these processes, be they executive or judicial, actually reflect what they intend.
$9B ‘Remedy’ to SCOTUS Ruling Still Leaves Patients Behind
In July 2023, the Centers for Medicare and Medicaid Services (CMS) issued a proposed ‘remedy’ to the United States Supreme Court’s 2022 ruling in American Hospital Association v. Becerra. In the case, AHA argued that the U.S. Department of Health and Human Services (HHS) misapplied statutory rulemaking powers when reducing certain payments to 340B hospitals from 2018 to 2022 because the federal agency had not completed a survey of those hospitals drug acquisition costs, as outlined in statute. HHS argued the interpretation of the statutory language should yield to their interpretation due to the precedent set by Chevron, which held that, generally speaking, courts and private actors should allow executive agencies to define the meaning in statutory language when it’s reasonably interpreted in multiple ways outside of those issues defined by legislators.
The idea behind Chevron Deference is that these agencies are generally responsible for making rules and enforcing them and courts don’t necessarily want to be bogged down by bad-faith claims on what certain statutory language actually means. In this instance, despite fears SCOTUS might overturn Chevron, the high court did not touch the precedent but did reject HHS’ claim regarding interpretation of the statutory language – a survey must be performed or based on the average sales price by drug manufacturers (with certain adjustments). The Court ordered HHS to review the ruling and issue a ‘remedy’ to fill the gap created by what the affected hospitals would have made from 2018 to 2022 rather than a setting a specific dollar amount like one might see in a civil judgment. CMS’ estimated dollar amount is just about $9 billion across affected hospitals and CMS is proposing to make those payments in one lump sum to the approximate 1,600 affected hospitals.
In an addendum to the proposed rule, the amount of money going to each hospital is listed out. The addendum has to be downloaded from the CMS website under the filename NPRM OPPS Remedy for 340B-Acquired Drug Payment Addendum AAA. A curious reader should note, the entities listed are by hospital, not by hospital system. There’s some really interesting tid bits in the spreadsheet worth pointing out.
To illustrate how lucrative hospitals view the 340B gravy train, let’s look at Cleveland Clinic. Cleveland Clinic will receive about $35 million in remedy payments, but in 2018 only had $74 or so in drug claims. If that makes your eyes pop, it should. The clinic went from about $10 million in drug claims in 2020 to almost $1.4 billion 2022. According to Cleveland Clinic’s 2021 tax return, the provider’s financial assistance ‘at cost’ (or ‘charity care’) as was about $177 million or about 1.5% of its total expenses.
Various Bon Secours hospitals will receive almost $50 million.
Ascension hospitals will receive about $45 million.
If those two hospital chains sound familiar, they should. Bon Secours was the subject of The New York Times article highlighting how the hospitals system abused 340B, specifically, by investing program revenues from poorer neighborhoods into richer communities. Dr. Lucas English, in the article, described the practice as “…laundering money through this poor hospital to its wealthy outposts.”
Ascension was the subject of another explosive NYT investigation for chronic understaffing in the name of decreasing expenses, even at the cost of patient safety. The whole design left the facilities in the system unprepared for the COVID-19 pandemic, which eventually lead to the system hiring contract and traveling medical staff in order to keep up – tossing the labor and wage ecosystem in healthcare into an upward spiral of costs which would be later passed onto patients by way of increased insurance premiums.
These aren’t the only two systems in the dataset that deserve scrutiny but they certainly represent a tiny sample of why the discount drug program needs reform.
Ultimately, the issue with these payments isn’t necessarily “hospitals shouldn’t get them” but “Why should bad-actors be further rewarded when they’ve already been shown to be abusing the program?”
Earlier this year, Kaiser Family Foundation analyzed charity care vs. realized tax breaks hospitals enjoy. Hospitals received about $28.1 billion in tax breaks while only dolling out about $16 billion in charity care – and that’s before the entirely non-transparent revenues generated from 340B. All of these entities are registered as “non-profit” but that $12 billion gap and stories like Bon Secours and Ascension and even the Cleveland Clinic’s leave even a casual viewer asking “But are they really?”
Earlier this month, Lown Institute’s Vikas Saini stated, “The distinction between not-for-profit and for-profit – certainly in health care and certain in relationship to hospitals – is negligible or nonexistent.”
SCOTUS wasn’t tasked with considering if hospitals that might receive a differential payment might not necessarily be operating within the intent of the 340B program but this kind of highlights why Congress can’t leave this issue to the courts to hammer out between the parties. Congress has a chance and, arguably the obligation, to make sure sufficient statutory provisions exist to actually meet achieve the intent of the program. Indeed, not acting to reform the program, in light of these “remedy” payments, will only encourage more bad actors to abuse 340B.
DEA Appears Open to Tele-Script for Certain Controlled Substances
On August 7th, the Drug Enforcement Agency (DEA) issued a notice of meeting regarding telemedicine prescription of certain controlled substances (Schedule II only). The meeting(s) will be conducted as “listening sessions”, conducted Tuesday, September 12th, and Wednesday, September 13th from 9 a.m. to 5:30 p.m. at the DEA Headquarters (located at 700 Army Navy Drive, Arlington, VA). Participants must pre-register using this link, before or on August 21st. In-person requests will be granted via lottery and the sessions will be live-streamed. Similarly, those wishing to provide limited oral presentation, either in-person or via live-stream must also fill out the form. Again, these will be selected by DEA personnel based upon quality of summary of presentation. Presentations may be made by anyone with an interest in and expertise in the subject matter. The DEA has asked for feedback on the following questions:
If telemedicine prescribing of schedule III–V medications were permitted in the absence of an in-person medical evaluation, what framework, including safeguards and data, with respect to telemedicine prescribing of schedule III–V medications do you recommend to help DEA ensure patient safety and prevent diversion of controlled substances?
Should telemedicine prescribing of schedule II medications never be permitted in the absence of an in-person medical evaluation? Are there any circumstances in which telemedicine prescribing of schedule II medications should be permitted in the absence of an in-person medical evaluation? If it were permitted, what safeguards with respect to telemedicine prescribing of schedule II medications specifically would you recommend to help DEA ensure patient safety and prevent diversion of controlled substances?
If practitioners are required to collect, maintain, and/or report telemedicine prescription data to DEA, what pieces of data should be included or excluded? What data is already reported to federal and state authorities, insurance companies, and other third parties?
If pharmacies are required to collect, maintain, and/or report telemedicine prescription data to DEA, what pieces of data should be included or excluded? What data is already reported to federal and state authorities, insurance companies, and other third parties?
The listening sessions come as a direct result of the DEA receiving truly unprecedented responses to proposed rules published in March, in anticipation of the end of the COVID-19 public health emergency, and, thus, the DEA’s telemedicine waiver issued at the beginning of the COVID-19 pandemic. In all, the DEA received almost 40,000 comments across the proposed rules with much focus on the General Telemedicine proposed rule. We covered the content of those rules (and why they were a step backward as written) in March. In particular, we expressed concern over returning to pre-pandemic limitations on telemedicine when the pandemic-related waiver did not prove any spike in diversion and did, indeed, improve access to medication assisted substance use treatment for many patients. Along similar lines, because testosterone is considered a controlled substance, such a return at the height of bias-driven, state-based legislation limiting access to certain gender affirming care would have a disproportionately harmful impact among transgender men and undermine President Biden’s commitment to combat these hateful efforts.
The relationship between the DEA and harm reduction advocates has been long and fraught. Many harm reduction advocates criticize the role of law enforcement’s actions, particularly that law enforcement agency, working against best practices in public health, even those best practices recognized by federal public health agencies. For example, a couple of weeks ago, we highlighted the Substance Abuse Mental Health Administration’s document (currently open to public comment) aimed at formalizing certain policy positions, entitled Harm Reduction Framework. Nowhere in the “framework” is the conflict with law enforcement positions addressed.
Putting more pressure still on the DEA’s absolute refusal to meet its commitment from 2009 to introduce meaningful telemedicine rules (in response to passage of the Ryan Haight Online Pharmacy Consumer Protection Act) is the fact that the stimulant medication shortage is at its worst. Things are so bad the U.S Food and Drug Administration (FDA) and DEA issued a joint letter on August 1st to further detail actions being taken to address the shortage and consumer struggles. The problem with the letter is it is largely bypasses the responsibility the DEA has in the current situation. Leaning into a claim that manufacturers haven’t filled their annual quota limits in production and pointing fingers at an increase in legitimate prescription of stimulants to manage conditions like attention deficit hyperactivity disorder (ADHD), the letter fails to recognize that the DEA also places extraordinary limits and scrutiny on pharmacies dispensing stimulant medications. Known as “drug diversion”, the idea behind monitoring pharmacies has some merit when viewed under the lens that pharmacies have a responsibility to limit index events like those “pill mills” associated with the opioid epidemic. However, the DEA doesn’t take responsibility for pharmacy raids or strict enforcement against prescribing providers working to keep patients from turning to street supplies by providing legitimate prescriptions.
For the DEA to be a meaningful partner in combatting both illicit and harmful drug use and overdoses and help to address drug shortages, limiting harmful diversion, the agency needs to consider a dramatic shift from an “all drugs are bad, and the people who use them are bad” mindset. There needs to be a thoughtful “medium, wherein stakeholders other than law enforcement can engage in distinguishing best practices in supply chain security and harm reduction and readily identifying criminals taking advantage of patients seeking care by any means they can achieve it – including illegal and illicit channels.
Patient advocates outside of harm reduction and substance use disorder focuses and the industry stakeholders who serve these patients would do well to consider engagement in these and other opportunities to help re-shape and re-imagine the DEA’s role, ideas, and programs to better serve the public at large, better secure the supply chain and limit disruptions, and ensure patients can have ready, reliable, and modernized access to the care we need.
Payers Finally Facing Scrutiny for Denying Coverage
In February of this year, we covered the issues of inequity and administrative barriers patients face when seeking medically necessary care, especially when that care is for chronic or complex conditions. The blog followed ProPublica’s review of Christopher McNaughton’s trials (quite literally – there was a lawsuit) and tribulations with United Healthcare’s refusal of coverage. The situation highlighted how the payer had never intended to cover McNaughton’s care, regardless of necessity, and shopped “appeals” doctors in order to avoid finding his care was “medically necessary” and therefore required to be covered.
The ProPublica article was published some two years after United Healthcare (UHC) had floated instituting a policy of retroactive review and denial of emergency room care – the scheme went something along the lines of “if we think you didn’t really need to go to the ER, we’ll make you pay the whole bill yourself.” The tactic was roundly shouted down by advocates and providers as dangerous. Afterall, a payor reviewing documents rather than actually serving in an emergency room is never going to grasp the details of certain situations – like the unique symptoms women face when having a heart attack. Eventually UHC pressed the pause button and after some jockeying back and forth between the payer and the American Hospital Association, UHC said the entity wouldn’t enact the policy. Turns out, that might not have been an honest assertation according to a lawsuit issued by the U.S. Department of Labor (DOL). To be clear, the payer entity that’s targeted in the suit is a third-party administrator within UHC’s subsidiary – called UMR – but arguing that nuance isn’t going to matter to a patient who had their care claim unjustly denied.
The lawsuit asserts that UMR denied “thousands” of urine drug screenings and emergency room visits and violated the Affordable Care Act’s “prudent layperson” standard. That standard requires that payers reviewing claims consider how the average patient might approach concerns or symptoms they’re experiencing, not a medical professional. Now, there’s a thing about when these denials took place, 2015 to 2018, means UHC’s proposed policy of retroactive denial might have been an improvement over their previous policy of denying every urinalysis claim. Which is just…wild. Further, the suit alleges that UHC wouldn’t clearly tell doctors what additional information they needed during appeals processes – which sounds strikingly related to McNaught’s troubles with the payer.
DOL wants UHC to review all denied claims and adopt new policies which wouldn’t result in what amounts to an automatic denial process. And it’s not unheard of. In 2020, a judge in California found another UHC subsidiary automatically denied coverage of care to patients seeking to use their mental health and substance use treatment benefits and ordered some 67,000 claims to be re-reviewed and new processing policies to be adopted.
Similarly, Cigna is coming under scrutiny. A class action lawsuit filed in California is alleging Cigna denied some 300,000 claims in just two months last year. The absolutely bonkers part about that is Cigna used an algorithm that spent just 1.2 seconds on each claims review before sending them off to doctors to sign them – meaning those claims might not have ever actually been seen by human eyes in what amounts to an automatic denial process. Cigna, for its part, decided its public facing comment would be to call ProPublica’s coverage of their denial process “poorly written”. All that despite the House found ProPublica’s investigation worthwhile enough to drag Cigna in front of the Energy and Commerce Committee for a hearing on the legality of these denials. Mike Kreidler, the insurance commissioner for Washington characterized Cigna’s operation as an “abhorrent” practice “to routinely deny just to enhance the bottom line.”
All of this coming just weeks after the Office of Inspector General released a report on how Medicaid managed care organizations (MCOs) are utilizing prior authorizations processes and denial of care in an abusive fashion, harming the poorest patients in the country and with little oversight by the states contracting these MCOs. Among those listed with a prior authorization denial rate higher than 25% was United Healthcare. And none of that touching that more and more providers are contracted by UHC, meaning those denials were denials of care in which their own providers had decided what was medically necessary.
The scrutiny of payors coming by way of lawsuits is welcomed but advocates and policymakers shouldn’t wait for judges to determine the scope of harm patients are experiencing. We need to seek a statutory and regulatory reigning-in of these run-away practices bilking our healthcare systems at the expense of patient health. And we need to do it now.
Feds: “Harm Reduction Framework”
On May 15th, Substance Abuse Mental Health Services Administration (SAMHSA) published a document which seeks to “…inform SAMHSA’s harm reduction activities moving forward, as well as related policies, programs, and practices,” and “to inform SAMHSA of opportunities to work with other federal, state, tribal, and local partners toward advancing harm reduction approaches, services, and programs.” The document, called the Harm Reduction Framework, specifically acts as a “level setting” document in addressing substance use as a public health issue.
While the document includes reach within the Office of National Drug Control Policy (ONDCP) and other agencies, like the Centers for Disease Control and Prevention (CDC), it does not have any “mission control” or enforceable policy influence with the Drug Enforcement Agency (DEA) or other law enforcement, which has been a central tool in federal, state, and local government responses to drug use and the opioid epidemic. Indeed, “law enforcement” only shows up once in the document’s contents and once more in the document’s references list. Arguably, whereas the document serves well as a “level setting” opportunity between various stakeholders, which claims to include “law enforcement” personnel, this effort is admirable but will lack “teeth” due to harm reduction as a programmatic idea from a public health lens when law enforcement remains a contraindicated method of response.
SAMHSA has also asked for specific feedback on the framework by way of a public comment form, indicating an effort more to formalize the framework's ideas as a policy stance.
The form follows the flow of the document with the first question seeking general and overall feedback. The second question asks for feedback on the document’s introduction and review of the working group, why the document exists, and historical recognition of how harm reduction has operated in response to substance use. This should be relatively uncontroversial for most respondents. The majority of feedback may seek to clarify or otherwise add details which lengthen the document, if adopted, but will not necessarily impact the substance of this section. The only area which might become “sticky” is the inclusion of “sex work” among “behaviors” associated with substance use and among those who might benefit from harm reduction programming.
The next four questions seek feedback on the core chaptered content pf the document as follows:
“Pillars” of harm reduction
“Supporting Principles”
“Core Practices”
“Community-Based Harm Reduction Programs” (CBHRP)
The document’s Pillars are outlined to include:
Guided by people who use drugs (PWUD) and with lived experience of drug use (this might also include family members, intimate partners, friends, and so forth of PWUD)
The inherent value of people
Commits to deep community building and engagement
Promotes equity, rights, and reparative social justice
Offers low-barrier access and non-coercive support
Focuses on any positive change as defined by the person
Supporting Principles include:
Respecting autonomy
Practicing acceptance and hospitality
Providing support
Connecting family (biological and chosen)
Provides many pathways to wellbeing across the continuum of health and social care
Values practice based evidence and on-the-ground experiences
Cultivates relationships
Assists and not directs
Promotes safety
Engages first
Prioritizes listening
Works toward systems change
Core Practice Areas” include:
Safer practices
Safer settings
Safer access to healthcare
Safer transitions to care
Sustainable workforce and field
Sustainable infrastructure
The final segment focuses on a brief description of CBHRPs, up to and including research projects used to explore innovation and efficacy of particular programs.
This section is noted with an asterisk “as permitted by law” – a nod toward the issue of law enforcement as a primary response tool to substance use and the limitations of SAMHSA as a result.
Advocates should anticipate some funding and program initiatives to reflect the general ideas around this framework or any final product around this framework. However, those barriers as a result of law enforcement and politicized attitudes will remain a barrier and perhaps present challenges for implementing novel programs. Strategically, much like SAMHSA’s drug court grants, the agency should consider how to leverage supportive funding incentives for states and municipalities to involve themselves in any resulting programming.
The public comment period is open until August 14th.
House Appropriations Mark-Up Alarms HIV Advocates
On July 14th, the U.S House of Representatives Committee on Labor, Health and Human Services, Education, and Related Agencies (LHHS) issued its fiscal year (FY) 2024 appropriations mark-up (Editor’s Note: the mark-up hearing can be viewed here). The bill passed out of committee on a party line vote, with Republicans voting in favor and Democrats voting against. The party split reflects what Democrats have framed as bad faith undermining of agreements on spending levels made in May’s debt limit ceiling deal and include policy provisions geared toward stoking a “culture” war. While the leading issue is incredibly steep cuts to public health programs, including entirely eliminating funding for the previous administration’s Ending the HIV Epidemic initiative (which the Biden Administration wished to continue), the policy riders at issue would also undermine best practices for achieving our public health goals by limiting the types of care, education, and support services communities accessing HIV services might be able to access.
Brass tax, Committee proposed:
Ending the HIV Epidemic eliminated (-$524M)
Ryan White HIV/AIDS Program reduced (-$74M, eliminates dental coverage and AIDS Education Training Centers [AETCs], as included in Part F)
Minority AIDS Initiative reduced (-$32M)
Substance Abuse Mental Services Administration – MAI eliminated (-$117M)
Flat funding for the Centers for Disease Control and Prevention’s HIV and HCV prevention programs
Flat funding for other parts of the Ryan White HIV/AIDS Program, particularly for AIDS Drug Assistance Programs (ADAPs)
Non-HIV related cuts seek to further defund public education and the National Institutes of Health and eliminate Title X and Teen Pregnancy Prevention Programs, the federal work-study program, among other things.
HIV advocates across the country decried the bill, including but not limited to The AIDS Institute, HIV + Hepatitis Institute, AIDS United, the National Association of State and Territorial AIDS Directors (NASTAD), and the National Minority AIDS Council. Southern AIDS Coalition (SAC) has organized a letter for individual advocates and organizations to sign-on. The letter is addressed to Senators Tammy Baldwin and Shelly Moore Capito. Astute observers already recognize this appropriation will not survive the Senate as drafted by the House.
Earlier this year, HIV media outlet The Body called these efforts out for what they are and by title “HIV is Being Swept Up in the Anti-Woke Agenda”. Mandisa Moore-ONeal, Executive Director of the Center for HIV Law and Policy (CHLP), drew a direct link between behavior seen earlier this year in Tennessee as a forebearer of federal moves, “Can’t help but think back to all those…who dismissed us when we said to notice these state funding cuts and to start moving against them but told us again and again ‘It’s just the South’.” The Centers for Disease Control & Prevention’s landing page for regional analysis on HIV identifies that 51% of new HIV diagnoses are among Southerners.
Politically, Southern states are often dismissed by politicians (and advocates) not from here because some of the country’s most conservative strategies are developed in the South. However, the racial diversity of the South is also the reason progressive advocates need to focus on the region. That same diversity is precisely why the nation’s civil rights battles started here and why the forefront of combatting the domestic HIV epidemic, be it advocacy, services, or policy, must be focused here.
The Community Access National Network has already joined our colleagues in signing-on and condemning these cuts which, if enacted would not only bring a halt toward our progress in combatting HIV and other public health concerns, but would signal a dramatic step back and make our goals impossible to achieve. The House’s appropriation amounts and the policy riders included are not only mean-spirited, political jockeying of resources the most vulnerable communities in the country rely upon, they are staggeringly dangerous.
New CDC Report; More than a Decade After a Cure, HepC Persists
Last year, the Centers for Disease Control and Prevention (CDC) published a Vital Signs report detailing how “too few people” are being “treated for Hepatitis C” (subtitled: “Reducing Barriers Can Increase Treatment and Save Lives”). Today, the CDC’s landing page reflects a finding from April 2020 that reads “dramatic increases in Hepatitis C” (subtitled: “CDC now recommends hepatitis C testing for all adults”). And in late June, the CDC published a new Morbidity and Mortality Weekly Report (MMWR) on a worryingly low rate of HCV clearance in the United States.
Our previous blog reviewed last year’s report under the lens of health disparities highlighted by researchers’ review of 48,000 patient charts that met the inclusion criteria for the analysis. Then, much like in this new report, identified that lack of curative treatment access was not uniform and was largely informed by the type of insurance patients qualified for. Those payer types (Medicaid, Medicare, and Commercial plans) also represent patients from different backgrounds – meaning different socio-economic statuses, different genders and racial backgrounds – with different outcomes. Overall, Medicaid recipients were only ever prescribed curative treatment about 23% of the time, whereas Commercial payer patients were able to see that rate increase to 35%. The CDC also recognized these payers, and the politicians who set the public policy of Medicaid, represent incredibly tangible barriers via administrative processes, like prior authorization, and policy barriers, like requiring sobriety, a high level of liver damage, or other restrictions to gaining access to curative treatments.
For this year’s report, researchers partnered with Quest Diagnostics to review the viral clearance (or cure) of approximately 1 million patients with an initial infection (Quest provided data for 1.7 million patients with evidence of a history of HCV during the direct acting agents era, or from January 1, 2013 – December 31, 2022). Based on an estimated 2.4 million people in the United States with HCV, this sample represents about 43% of those believed to have experienced an HCV infection in this time frame. This is noted as a limitation in the data, in part, because it only represents data from one commercial laboratory. Though, reasonable observers can make certain conclusions from this data.
Now, we should also note, only about 88% of the 1.7 million patients identified as having evidence of HCV infection ever had received testing and, of those, 69% were identified as having an initial infection. This means the majority of patients identified were newly diagnosed and not facing a chronic HCV infection. Of those, about 7% of patients showed evidence of viral persistence.
Authors note “These findings reveal substantial missed opportunities to diagnose, treat, and prevent Hepatitis C in the United States.”
Coverage was highest among those enrolled in commercial insurance (50%) and lowest in Medicare and Medicaid (8% and 9%, respectively). Particularly startling in the differences between payer types was the prevalence of viral testing; those with an unspecified payor type were screened at about 79% and those with commercial insurance or Medicare had a testing prevalence of about 91%.
Patients with “other”, “unspecified”, or Medicaid as their insurance or payer had showed a lower viral clearance rate (23%, 33% and 31% respectively) than their counterparts enrolled in Medicare or commercial plans (40% and 45%, respectively). Overall, the cure rate was about 34%.
The age range with the highest rate of HCV diagnoses was 40-59 years, representing about 43% of the patient records reviewed. 60% were identified in their charts as male. However, the highest rate of viral clearance was among those aged over 60 and the lowest was for those aged between 20-29 years.
Other limitations to the data include a lack of uniformity in the follow-up period between testing, which might lead to some difference in rates. Similarly, patients might use or be referred to a different lab for follow-ups. Though, the data also does not follow patients and would not capture any representation of subsequent reinfection and cannot make any assumptions as to clearance or viral persistence among those who did not have RNA testing (and referral for treatment) – meaning the data likely underestimates the patients in each of these categories.
Advocates can look toward these data and findings to inform necessary policy changes, particularly by payer type and in seeking appropriate provider activation on screening and treatment. The sheer reality is HCV is both preventable and curable and policymakers and payers need to work more efficiently in order to prevent the approximate 14,000 HCV related deaths this country faces annually.
Prescription Drug Advisory Boards: What They Are and Why They Matter to Patients
It’s no secret that the high cost of healthcare is a significant concern for most Americans. The total national health expenditure in 2021 increased by 2.7% from the previous year to 4.3 trillion dollars which was 18.3% of the gross domestic product. The federal government held the majority of the spending burden at 34%, with individual households a close second at 27%. A cornerstone component of medical treatment is the access to prescription drugs. In 2019 in the U.S., the government and private insurers spent twice as much on prescription drugs as in other comparatively wealthy countries. Despite catchy phrases that poll well, and “simple” solutions by politicians that promise to fix the problem—such as Prescription Drug Advisory Boards (also known as Drug Pricing Advisory Boards)—it is mindful to remember one thing: if it sounds good to be true, then it probably isn’t true.
CANN PDAB infographic: What are they and why do they matter? (https://tiicann.org/dpab-project.html)
While list prices of prescription drugs continue to increase, medication costs do not represent the largest share of healthcare costs or the largest growth in healthcare costs in the United States. The cost burden on patients is so untenable for many that some have to decide between paying for medications, food, or mortgages. However, due to a number of incentives and the role of loosely regulated pharmacy benefit managers (PBMs), there is little direct relationship between drug list prices and patient cost burdens. This fact is only just now being appreciated by lawmakers but is not currently reflected in our healthcare funding schemes. As such, the discourse surrounding lowering cost is a consistently turbulent sea navigated by diverse public and private parties, with the language around drug pricing assuming efforts to curb costs relate to patient costs and access – but not explicitly saying so (and for good reason). Some proposals are government related, such as federal drug pricing proposals. Recent developments are state-level focused closer to home. One such development is the Prescription Drug Advisory Boards, or PDABs.
PDABs are part of state divisions of insurance. Drug pricing efforts, in the general sense, could be a good thing. PDABs are being marketed to the public as a better means to make drugs more affordable for patients. However, the details of the implementation of developing PDABs are wherein lies significant challenges. Overall, the boards focus specifically on the prices of the drugs. However, the focus on pricing is mainly related to what governments, insurance companies, hospitals, and pharmacies are paying for the medications. This purview and the monitored metrics associated with PDABs do not necessarily translate into the actual costs patients pay at the pharmacy counter.
Because these designs are singularly focused on the “cost” to payors, current proposals and initiatives benefit both public and private payors at the expense of the patient access and the provider-patient relationship. It is unacceptable for any planned PDAB activity to disrupt the patient-provider relationship. Community Access National Network (CANN) has consistently opposed any policy initiative that might increase administrative barriers and patient burdens. Two examples are step-therapy and prior authorization. Activities such as these are considered what is known as utilization management. Utilization management helps lower prescription drug spending for public and private payors but creates additional costs for patients financially and logistically, affecting their continuity of care, amounting to a cost burden shift, not a meaningful increase of access to affordable, high-quality care and treatment for patients.
Additionally, the narrow specific focus on the list prices of drugs overlooks essential issues. Lowering the list price for medications can, for example, harm organizations that depend on revenues from the 340B Drug Pricing Program. The 340B program allows safety net clinics and organizations to purchase prescription drugs from manufacturers at a discounted price while being reimbursed by insurance carriers at a non-discounted cost. The surplus enables these entities to provide many services that the low-income populations they serve depend on. This is especially vital to low-income people living with HIV that do not have the means to afford all of their healthcare needs.
It is imperative that PDABs receive input directly from patients and caregivers as well. PDABs are aggregating a large amount of data. However, more of that data needs to include considerations of the patient experience. For example, drug rebate reductions can impact care and support services, such as transportation assistance or mental health services at federally qualified health centers (FQHCs). Moreover, there needs to be an examination of the actual pass-through savings to patients. Most importantly, PDABs need to explore how pricing decisions affect patient access. A lower drug list price is not beneficial to patients if it creates or increases administrative burdens or increases costs for patients in other ways outside of paying for the cost of medication.
Most policymakers do not always have robust experience in understanding the nuances of dealing with public health programs, clinics, and populations. This is especially true regarding the marginalized community of people living with or at risk for acquiring HIV, those affected by Hepatitis C, or people who use drugs. PDABS must be held accountable for acquiring anecdotal qualitative and quantitative data regarding patient experience, accessibility, and affordability while developing recommendations related to drug pricing. As it stands, of the states that have implemented a PDAB, none have statutorily mandated metrics monitoring patient experience and access.
Patients, caregivers, and advocates with direct experience and greater understanding of the policy landscape around healthcare access play a vital role in helping to shape legislation and informing proper implementation of programs to meet the goals those programs were “sold” on. If monitored metrics do not consider or reflect patient experiences, then the program is simply not about increasing access for patients.
PDABs, fortunately, do have numerous opportunities for patients, caregivers, advocates, and providers to become involved and to elevate patient priorities over that of other stakeholders. Getting involved and staying involved with a state’s PDAB work is critically necessary to ensure any final work or regulation is patient-focused.
CANN will be present and offering feedback at various PDAB meetings in affected states. The next meeting CANN will be attending is virtual for the state of Colorado, on July 13th at 10am Mountain time. You can register here and participate in ensuring any action taken reflects patient needs.
Opioid Settlements in America
In 2021, a group of state State Attorneys General announced a $26-billion national opioid settlement with three of the largest drug distributors—McKesson Corp., Cardinal Health, Inc., and AmerisourceBergen Corp.—and drugmaker Johnson & Johnson that would see $21b and $5b from those groups, respectively. This settlement deal was approved by all but four states—Alabama, Oklahoma, West Virginia, and Washington—and distribution of those funds began in 2022.
As with every such settlement, funds are distributed to states and municipalities, but how those funds are used is largely up to the recipients. One of the primary critiques of the 1998 Tobacco Master Settlement Agreement was that states and municipalities could use the settlement funds for any purpose. Many states, including West Virginia, used (and abused) those funds to plug holes in their budgets, fund infrastructure improvements, and, in the case of West Virginia, fund teacher pension funds. This lack of direction and oversight meant that relatively few funds actually went to provide healthcare, cessation, or prevention services. Despite this, adult smoking rates have largely plummeted since 1998 but remain relatively high in Appalachian and Midwestern states (Figure 1).
Figure 1 – Current Cigarette Use Among Adults (Behavior Risk Factor Surveillance System) 2019
Source: Centers for Disease Control and Prevention. (2021, October 22). Map of Current Cigarette Use Among Adults. https://www.cdc.gov/statesystem/cigaretteuseadult.html
Negotiators appear to have learned from that mistake, and the National Opioid Settlement is different. However, while the settlement agreement requires that 85% of the funds going directly to states and municipalities must be used for “…abatement of the opioid epidemic,” the settlement doesn’t go so far as to enumerate what qualifies as “abatement.”
According to Kaiser Family Foundation reporting, funds from the settlement are split between states and are then divided in varying percentages across state agencies, local governments, and councils that oversee opioid abatement trusts. But, there has been little transparency around the settlements, including how much each state is received, how those funds are then divided, and how those funds will be used. To date, just 15 states (Arizona, Colorado, Connecticut, Delaware, Florida, Idaho, Massachusetts, Minnesota, Missouri, New Hampshire, New Jersey, North Carolina, Oregon, South Carolina, and Utah) have explicitly promised to report 100% of their Distributor and Johnson & Johnson settlement expenditures. These promises are not, however, legally binding, and recent moves by certain state administrative and legislative bodies to make private information that is statutorily mandated to be public under state Sunshine Laws bode poorly for those hoping to hold states accountable for their use of the funds.
There have been some successful efforts to track the distribution of national settlement funding out of the National Academy for State Health Policy (NASHP). Their map, however, is limited in that it only covers funds from that National Opioid Settlement, meaning that states that chose to continue pursuing additional damages (i.e., West Virginia, Alabama, Oklahoma, and Washington) are not tracked on the map. In addition to the interact map, NASHP provides a pretty comprehensive breakdown of how each state is using opioid settlement funds, including those states that did not participate in the national settlement.
Because we’re still in the early days of the settlement disbursements, there’s not really a great way to measure whether or not the funds will be successfully utilized, nor whether or not the abatement programs will actually have an impact. What we have seen outside of the settlement is that there is little consensus between states on how best to approach the continuing opioid epidemic. While some states increased access to harm reduction services, others have reduced access to, heavily regulated, or eliminated those services altogether.
In states where the opioid epidemic has become part of the fabric of life, such as Indiana, Kentucky, Ohio, and West Virginia, anecdotal reports and some limited research have found that, while additional and public health professionals are actively attempting to implement policies, plans, and interventions to openly and positively confront the opioid crisis, state residents are simply exhausted after dealing with over twenty years of devastation, loss, and both perceived and real destruction to their ways of life.
On the anecdotal front, I recently returned to Eleanor, WV, where I briefly lived and attended school and where my father taught music. What I found made my heart ache: the town’s lone shopping center essentially abandoned and left in disrepair; a middle school whose track and football field (which are still actively used by students) so destroyed that the track was little more than broken concrete loosely interspersed between fields of overgrown grass and bleachers literally tilting from rust and overuse; a set of buildings that once served as some of the town’s few apartment buildings literally burned out and boarded up with graffiti-covered plyboard; school bus stops that looked like they’d been hit by a tornado, and nobody repaired them.
We’re not talking about a major metropolitan area—we’re talking about a town of just over 1,500 residents that carries the moniker, “The Cleanest Town in West Virginia.” And the devastation didn’t stop there. I spent the next hour or so driving along the routes I used to travel as a kid and teenager, and every place that once held a great memory for me was absolutely destroyed or so badly damaged as to be wholly unrecognizable.
When I go through Facebook to check on friends from my time in Eleanor, a significant percentage of them are either recovering, in active use, or have lost their lives to the opioid epidemic. Even after working in the public health and advocacy space for more than fifteen years, the realities sometimes feel remote, and this drive allowed me to see what the opioid epidemic has done to West Virginia: it has deprived us of hope that things can or will get better. What’s more, our state legislature seems determined to make things worse.
These are the states and towns where opioid settlement funds are desperately needed, but it’s unclear whether or not they will receive or utilize them well. I know that I will be actively following how these funds are used in Appalachia, particularly in my capacity as Founder and Executive Director of the Appalachian Learning Initiative (APPLI, pronounced like “apply”).
When Anti-Equality Means Anti-HIV
As a teenager in Western Illinois, the educational content I received on sexual health consisted of what can best be described as “MadLibs Sex Ed” - sheet after sheet of entire sections of a book, meticulously handwritten by my teacher with various words replaced by blanks. It stands to reason then that my functional knowledge of this content also had some gaps. In the years since, my world has drastically shifted as has the world around me. I came out, transitioned, and found my footing as a queer and trans health advocate, all the while access to comprehensive sex ed has remained polarized despite gains made in HIV prevention and treatment such as PrEP and U=U (Undetectable=Untransmittable). It is amongst this backdrop that I learned that the great State of Iowa had recently put a sweeping bill into law - SF 496 - that, under the guise of parental rights, eliminates the requirement of age-appropriate information about HIV/AIDS and HPV.
To say this is concerning is an understatement. By eliminating the mandate of HIV education, the state of Iowa is essentially choosing the path of willful ignorance on behalf of its young people. The State of Iowa would have one believe that young people are either too young to safely know about sexuality or are certain to have a parental figure able to competently have “the talk” with them in such a way that the necessary content will be covered. The reality is that the Centers For Disease Control and Prevention’s (CDC’s) Youth Risk Behavior Survey from 2021 found that 1 in 5 high school students is currently sexually active, and among those, only 52% used a condom the last time they had sex and less than 7% had been tested for HIV or STIs. Additionally, In the United States, 20% of new HIV diagnoses in 2020 were among young people aged 13-24. Earlier this month, Iowa Public Radio noted that despite national new HIV infection rates falling overall, Iowa’s new infection rates have remained steadfast. It would seem that now is not the time to stand down on HIV education efforts.
Truly comprehensive sex education empowers individuals with accurate and evidence-based information about HIV transmission, prevention, and treatment. By teaching individuals about how HIV is transmitted, the importance of condom use, regular HIV testing, and the use of PrEP and nPEP, comprehensive sex education equips folks with practical skills to protect themselves and their partners from HIV transmission and unintended pregnancy. Additionally and equally importantly, comprehensive sex ed, when done well, stresses the importance of communication, negotiation, and consent, which are essential elements in fostering healthy relationships throughout the lifespan and even outside of sexuality-based contexts.
It is also important to note that the same law that eliminates the requirement of HIV education also eliminates any mention of sexual orientation or gender identity through the sixth grade. As a queer and trans person raised in a similar environment, I spent my childhood and adolescence staring out across the corn and soybean fields, intensely aware that I was different yet with no feasible outlet (or Internet) to understand why. This bill (and the onslaught of others like it elsewhere in the U.S.) targets the exploration of sexual orientation and gender identity and hopes to harken back to the bad ol’ times when access to information was limited and supportive resources were scarce. Interestingly, this bill also includes mechanisms to make it easier to ban books in schools and explicitly prohibits students from serving on committees that determine or provide recommendations concerning banning books and other materials from school libraries. Because not only do we want young people under-informed about their bodies and their options, we also want to make sure they don’t dare have agency about what happens in their school - yet we hope for them to be good and engaged citizens. How interesting and perfectly sensible!
What happens next is both predictable (in that some of us have lived it before) and preventable. In the Mad Lib, SF 496 of it all, young people will not get access to the information they need to make sound decisions or to feel less isolated. There will still be gay young people or trans young people - just less understood by their peers and less likely to see themselves in the literature at their schools. There will be more young people who become HIV positive and unknowingly transmit it to others, and because removal of HIV education means removal of HIV testing information, there will be more people who have no idea about what testing means, how frequently to do it, and where one can do it. Therein lies the work for HIV advocates. The State of Iowa has created a “tough row to hoe” as they say in my hometown, but I take solace in the fact that whether overlooking the cornfields, the beaches of my adopted home, or the haze of a city elsewhere in the world, HIV advocates don’t give in and they don’t give up.